Modification of the hepatitis B virus envelope protein glycosylation pattern interferes with secretion of viral particles, infectivity, and susceptibility to neutralizing antibodies
- PMID: 24899172
- PMCID: PMC4136284
- DOI: 10.1128/JVI.01161-14
Modification of the hepatitis B virus envelope protein glycosylation pattern interferes with secretion of viral particles, infectivity, and susceptibility to neutralizing antibodies
Abstract
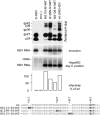
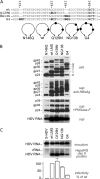
The envelope proteins of hepatitis B virus (HBV) bear an N-linked glycosylation site at N146 within the immunodominant a-determinant in the antigenic loop (AGL) region. This glycosylation site is never fully functional, leading to a nearly 1/1 ratio of glycosylated/nonglycosylated isoforms in the viral envelope. Here we investigated the requirement for a precise positioning of N-linked glycan at amino acid 146 and the functions associated with the glycosylated and nonglycosylated isoforms. We observed that the removal of the N146 glycosylation site by mutagenesis was permissive to envelope protein synthesis and stability and to secretion of subviral particles (SVPs) and hepatitis delta virus (HDV) virions, but it was detrimental to HBV virion production. Several positions in the AGL could substitute for position 146 as the glycosylation acceptor site. At position 146, neither a glycan chain nor asparagine was absolutely required for infectivity, but there was a preference for a polar residue. Envelope proteins bearing 5 AGL glycosylation sites became hyperglycosylated, leading to an increased capacity for SVP secretion at the expense of HBV and HDV virion secretion. Infectivity-compatible N-glycosylation sites could be inserted at 3 positions (positions 115, 129, and 136), but when all three positions were glycosylated, the hyperglycosylated mutant was substantially attenuated at viral entry, while it acquired resistance to neutralizing antibodies. Taken together, these findings suggest that the nonglycosylated N146 is essential for infectivity, while the glycosylated form, in addition to its importance for HBV virion secretion, is instrumental in shielding the a-determinant from neutralizing antibodies.
Importance: At the surface of HBV particles, the immunodominant a-determinant is the main target of neutralizing antibodies and an essential determinant of infectivity. It contains an N-glycosylation site at position 146, which is functional on only half of the envelope proteins. Our data suggest that the coexistence of nonglycosylated and glycosylated N146 at the surface of HBV reflects the dual function of this determinant in infectivity and immune escape. Hence, a modification of the HBV glycosylation pattern affects not only virion assembly and infectivity but also immune escape.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Analysis of the cytosolic domains of the hepatitis B virus envelope proteins for their function in viral particle assembly and infectivity.J Virol. 2006 Dec;80(24):11935-45. doi: 10.1128/JVI.00621-06. Epub 2006 Oct 4. J Virol. 2006. PMID: 17020942 Free PMC article.
-
Role of N glycosylation of hepatitis B virus envelope proteins in morphogenesis and infectivity of hepatitis delta virus.J Virol. 2003 May;77(9):5519-23. doi: 10.1128/jvi.77.9.5519-5523.2003. J Virol. 2003. PMID: 12692255 Free PMC article.
-
Support of the infectivity of hepatitis delta virus particles by the envelope proteins of different genotypes of hepatitis B virus.J Virol. 2014 Jun;88(11):6255-67. doi: 10.1128/JVI.00346-14. Epub 2014 Mar 19. J Virol. 2014. PMID: 24648462 Free PMC article.
-
Glycan Shielding and Modulation of Hepatitis C Virus Neutralizing Antibodies.Front Immunol. 2018 Apr 27;9:910. doi: 10.3389/fimmu.2018.00910. eCollection 2018. Front Immunol. 2018. PMID: 29755477 Free PMC article. Review.
-
Hepatitis B Virus and Hepatitis D Virus Entry, Species Specificity, and Tissue Tropism.Cold Spring Harb Perspect Med. 2015 Aug 3;5(8):a021378. doi: 10.1101/cshperspect.a021378. Cold Spring Harb Perspect Med. 2015. PMID: 26238794 Free PMC article. Review.
Cited by
-
Impact of Protein Glycosylation on the Design of Viral Vaccines.Adv Biochem Eng Biotechnol. 2021;175:319-354. doi: 10.1007/10_2020_132. Adv Biochem Eng Biotechnol. 2021. PMID: 32935143 Review.
-
A recombinant human immunoglobulin with coherent avidity to hepatitis B virus surface antigens of various viral genotypes and clinical mutants.PLoS One. 2020 Aug 13;15(8):e0236704. doi: 10.1371/journal.pone.0236704. eCollection 2020. PLoS One. 2020. PMID: 32790777 Free PMC article.
-
Analysis of the Physicochemical Properties, Replication and Pathophysiology of a Massively Glycosylated Hepatitis B Virus HBsAg Escape Mutant.Viruses. 2021 Nov 22;13(11):2328. doi: 10.3390/v13112328. Viruses. 2021. PMID: 34835134 Free PMC article.
-
Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network.Trends Microbiol. 2015 Dec;23(12):789-798. doi: 10.1016/j.tim.2015.08.007. Epub 2015 Oct 1. Trends Microbiol. 2015. PMID: 26439298 Free PMC article. Review.
-
Hepatitis B Virus Exploits ERGIC-53 in Conjunction with COPII to Exit Cells.Cells. 2020 Aug 12;9(8):1889. doi: 10.3390/cells9081889. Cells. 2020. PMID: 32806600 Free PMC article.
References
-
- Stirk HJ, Thornton JM, Howard CR. 1992. A topological model for hepatitis B surface antigen. Intervirology 33:148–158 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

