Quantitative proteomic analysis of the influenza A virus nonstructural proteins NS1 and NS2 during natural cell infection identifies PACT as an NS1 target protein and antiviral host factor
- PMID: 24899174
- PMCID: PMC4136281
- DOI: 10.1128/JVI.00830-14
Quantitative proteomic analysis of the influenza A virus nonstructural proteins NS1 and NS2 during natural cell infection identifies PACT as an NS1 target protein and antiviral host factor
Abstract
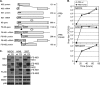
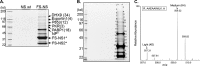
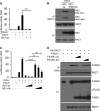
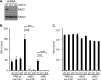
Influenza A virus (IAV) replication depends on the interaction of virus proteins with host factors. The viral nonstructural protein 1 (NS1) is essential in this process by targeting diverse cellular functions, including mRNA splicing and translation, cell survival, and immune defense, in particular the type I interferon (IFN-I) response. In order to identify host proteins targeted by NS1, we established a replication-competent recombinant IAV that expresses epitope-tagged forms of NS1 and NS2, which are encoded by the same gene segment, allowing purification of NS proteins during natural cell infection and analysis of interacting proteins by quantitative mass spectrometry. We identified known NS1- and NS2-interacting proteins but also uncharacterized proteins, including PACT, an important cofactor for the IFN-I response triggered by the viral RNA-sensor RIG-I. We show here that NS1 binds PACT during virus replication and blocks PACT/RIG-I-mediated activation of IFN-I, which represents a critical event for the host defense. Protein interaction and interference with IFN-I activation depended on the functional integrity of the highly conserved RNA binding domain of NS1. A mutant virus with deletion of NS1 induced high levels of IFN-I in control cells, as expected; in contrast, shRNA-mediated knockdown of PACT compromised IFN-I activation by the mutant virus, but not wild-type virus, a finding consistent with the interpretation that PACT (i) is essential for IAV recognition and (ii) is functionally compromised by NS1. Together, our data describe a novel approach to identify virus-host protein interactions and demonstrate that NS1 interferes with PACT, whose function is critical for robust IFN-I production.
Importance: Influenza A virus (IAV) is an important human pathogen that is responsible for annual epidemics and occasional devastating pandemics. Viral replication and pathogenicity depends on the interference of viral factors with components of the host defense system, particularly the type I interferon (IFN-I) response. The viral NS1 protein is known to counteract virus recognition and IFN-I production, but the molecular mechanism is only partially defined. We used a novel proteomic approach to identify host proteins that are bound by NS1 during virus replication and identified the protein PACT, which had previously been shown to be involved in virus-mediated IFN-I activation. We find that NS1 prevents PACT from interacting with an essential component of the virus recognition pathway, RIG-I, thereby disabling efficient IFN-I production. These observations provide an important piece of information on how IAV efficiently counteracts the host immune defense.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

