The gammaherpesviruses Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response
- PMID: 24899179
- PMCID: PMC4136288
- DOI: 10.1128/JVI.00841-14
The gammaherpesviruses Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response
Abstract
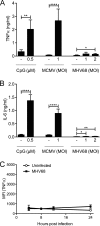
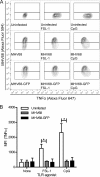
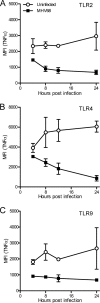
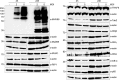
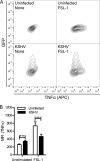
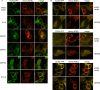
The human pathogen Kaposi's sarcoma-associated herpesvirus (KSHV), the etiological agent of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease, establishes lifelong latency upon infection. Murine gammaherpesvirus 68 (MHV68) is a well-established model for KSHV. Toll-like receptors (TLRs) play a crucial role for the innate immune response to pathogens. Although KSHV and MHV68 are detected by TLRs, studies suggest they modulate TLR4 and TLR9 signaling, respectively. In this study, we show that in bone marrow-derived macrophages (BMDMs), MHV68 did not induce a detectable proinflammatory cytokine response. Furthermore, MHV68 abrogated the response to TLR2, -4, -7, and -9 agonists in BMDMs. Similarly to observations with MHV68, infection with KSHV efficiently inhibited TLR2 signaling in THP-1 monocytes. Using a KSHV open reading frame (ORF) library, we found that K4.2, ORF21, ORF31, and the replication and transcription activator protein (RTA)/ORF50 inhibited TLR2-dependent nuclear factor kappa B (NF-κB) activation in HEK293 TLR2-yellow fluorescent protein (YFP)- and Flag-TLR2-transfected HEK293T cells. Of the identified ORFs, RTA/ORF50 strongly downregulated TLR2 and TLR4 signaling by reducing TLR2 and TLR4 protein expression. Confocal microscopy revealed that TLR2 and TLR4 were no longer localized to the plasma membrane in cells expressing RTA/ORF50. In this study, we have shown that the gammaherpesviruses MHV68 and KSHV efficiently downmodulate TLR signaling in macrophages and have identified a novel function of RTA/ORF50 in modulation of the innate immune response.
Importance: The Toll-like receptors (TLRs) are an important class of pattern recognition receptors of the innate immune system. They induce a potent proinflammatory cytokine response upon detection of a variety of pathogens. In this study, we found that the gammaherpesviruses murine gammaherpesvirus 68 (MHV68) and Kaposi's sarcoma-associated herpesvirus (KSHV) efficiently inhibit the TLR-mediated innate immune response. We further identified the KSHV-encoded replication and transcription activator protein (RTA) as a novel modulator of TLR signaling. Our data suggest that the gammaherpesviruses MHV68 and KSHV prevent activation of the innate immune response by targeting TLR signaling.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Blaskovic D, Stancekova M, Svobodova J, Mistrikova J. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468. - PubMed
-
- Knipe DM, Howley PM. 2013. Fields virology, 6th ed, vol II Wolters Kluwer, Philadelphia, PA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

