Maturation and vesicle-mediated egress of primate gammaherpesvirus rhesus monkey rhadinovirus require inner tegument protein ORF52
- PMID: 24899183
- PMCID: PMC4136283
- DOI: 10.1128/JVI.01502-14
Maturation and vesicle-mediated egress of primate gammaherpesvirus rhesus monkey rhadinovirus require inner tegument protein ORF52
Abstract
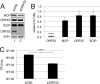
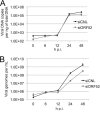
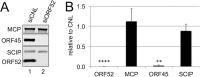
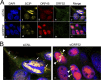
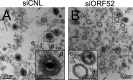
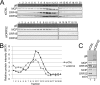
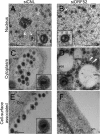
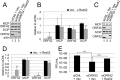
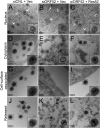
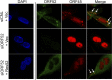
The tegument layer of herpesviruses comprises a collection of proteins that is unique to each viral species. In rhesus monkey rhadinovirus (RRV), a close relative of the human oncogenic pathogen Kaposi's sarcoma-associated herpesvirus, ORF52 is a highly abundant tegument protein tightly associated with the capsid. We now report that ORF52 knockdown during RRV infection of rhesus fibroblasts led to a greater than 300-fold reduction in the viral titer by 48 h but had little effect on the number of released particles and caused only modest reductions in the levels of intracellular viral genomic DNA and no appreciable change in viral DNA packaging into capsids. These data suggested that the lack of ORF52 resulted in the production and release of defective particles. In support of this interpretation, transmission electron microscopy (TEM) revealed that without ORF52, capsid-like particles accumulated in the cytoplasm and were unable to enter egress vesicles, where final tegumentation and envelopment normally occur. TEM also demonstrated defective particles in the medium that closely resembled the accumulating intracellular particles, having neither a full tegument nor an envelope. The disruption in tegument formation from ORF52 suppression, therefore, prevented the incorporation of ORF45, restricting its subcellular localization to the nucleus and appearing, by confocal microscopy, to inhibit particle transport toward the periphery. Ectopic expression of small interfering RNA (siRNA)-resistant ORF52 was able to partially rescue all of these phenotypic changes. In sum, our results indicate that efficient egress of maturing virions and, in agreement with studies on murine gammaherpesvirus 68 (MHV-68), complete tegumentation and secondary envelopment are dependent on intact ORF52.
Importance: The tegument, or middle layer, of herpesviruses comprises both viral and cellular proteins that play key roles in the viral life cycle. A subset of these proteins is present only within members of one of the three subfamilies (alphaherpesviruses, betaherpesviruses, or gammaherpesviruses) of Herpesviridae. In this report, we show that the gammaherpesvirus-specific tegument protein ORF52 is critical for maturation of RRV, the closest relative of Kaposi's sarcoma-associated herpesvirus (KSHV) (a human cancer-causing pathogen) that has undergone this type of analysis. Without ORF52, the nascent subviral particles are essentially stuck in maturation limbo, unable to acquire the tegument or outer (envelope) layers. This greatly attenuates infectivity. Our data, together with earlier work on a murine homolog, as well as a more distantly related human homolog, provide a more complete understanding of how early protein interactions involving virus-encoded tegument proteins are critical for virus assembly and are also, therefore, potentially attractive therapeutic targets.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
A Conserved Leucine Zipper Motif in Gammaherpesvirus ORF52 Is Critical for Distinct Microtubule Rearrangements.J Virol. 2017 Aug 10;91(17):e00304-17. doi: 10.1128/JVI.00304-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28615210 Free PMC article.
-
Murine gammaherpesvirus 68 ORF52 encodes a tegument protein required for virion morphogenesis in the cytoplasm.J Virol. 2007 Sep;81(18):10137-50. doi: 10.1128/JVI.01233-06. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634243 Free PMC article.
-
The Interaction between Tegument Proteins ORF33 and ORF45 Plays an Essential Role in Cytoplasmic Virion Maturation of a Gammaherpesvirus.J Virol. 2022 Nov 23;96(22):e0107322. doi: 10.1128/jvi.01073-22. Epub 2022 Oct 27. J Virol. 2022. PMID: 36300940 Free PMC article.
-
Nuclear Egress of Herpesviruses: The Prototypic Vesicular Nucleocytoplasmic Transport.Adv Virus Res. 2016;94:81-140. doi: 10.1016/bs.aivir.2015.10.002. Epub 2016 Jan 29. Adv Virus Res. 2016. PMID: 26997591 Review.
-
Comprehensive Analysis of the Tegument Proteins Involved in Capsid Transport and Virion Morphogenesis of Alpha, Beta and Gamma Herpesviruses.Viruses. 2023 Oct 6;15(10):2058. doi: 10.3390/v15102058. Viruses. 2023. PMID: 37896835 Free PMC article. Review.
Cited by
-
Comprehensive Analyses of Intraviral Epstein-Barr Virus Protein-Protein Interactions Hint Central Role of BLRF2 in the Tegument Network.J Virol. 2022 Jul 27;96(14):e0051822. doi: 10.1128/jvi.00518-22. Epub 2022 Jul 11. J Virol. 2022. PMID: 35862711 Free PMC article.
-
A survey of the interactome of Kaposi's sarcoma-associated herpesvirus ORF45 revealed its binding to viral ORF33 and cellular USP7, resulting in stabilization of ORF33 that is required for production of progeny viruses.J Virol. 2015 May;89(9):4918-31. doi: 10.1128/JVI.02925-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694600 Free PMC article.
-
A Conserved Leucine Zipper Motif in Gammaherpesvirus ORF52 Is Critical for Distinct Microtubule Rearrangements.J Virol. 2017 Aug 10;91(17):e00304-17. doi: 10.1128/JVI.00304-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28615210 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Inhibitor of cGAS (KicGAS), Encoded by ORF52, Is an Abundant Tegument Protein and Is Required for Production of Infectious Progeny Viruses.J Virol. 2016 May 12;90(11):5329-5342. doi: 10.1128/JVI.02675-15. Print 2016 Jun 1. J Virol. 2016. PMID: 27009954 Free PMC article.
-
Conserved linear motif within the immediate early protein ORF45 promotes its engagement with KSHV lytic cycle-promoting forkhead transcription factors, FOXK1 and FOXK2.J Virol. 2024 Oct 22;98(10):e0088624. doi: 10.1128/jvi.00886-24. Epub 2024 Sep 17. J Virol. 2024. PMID: 39287387 Free PMC article.
References
-
- Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708–2714 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

