Infection and pathogenesis of canine, equine, and human influenza viruses in canine tracheas
- PMID: 24899186
- PMCID: PMC4136294
- DOI: 10.1128/JVI.00887-14
Infection and pathogenesis of canine, equine, and human influenza viruses in canine tracheas
Abstract
Influenza A viruses (IAVs) can jump species barriers and occasionally cause epidemics, epizootics, pandemics, and panzootics. Characterizing the infection dynamics at the target tissues of natural hosts is central to understanding the mechanisms that control host range, tropism, and virulence. Canine influenza virus (CIV; H3N8) originated after the transfer of an equine influenza virus (EIV) into dogs. Thus, comparing CIV and EIV isolates provides an opportunity to study the determinants of influenza virus emergence. Here we characterize the replication of canine, equine, and human IAVs in the trachea of the dog, a species to which humans are heavily exposed. We define a phenotype of infection for CIV, which is characterized by high levels of virus replication and extensive tissue damage. CIV was compared to evolutionarily distinct EIVs, and the early EIV isolates showed an impaired ability to infect dog tracheas, while EIVs that circulated near the time of CIV emergence exhibited a CIV-like infection phenotype. Inoculating dog tracheas with various human IAVs (hIAVs) showed that they infected the tracheal epithelium with various efficiencies depending on the virus tested. Finally, we show that reassortant viruses carrying gene segments of CIV and hIAV are viable and that addition of the hemagglutinin (HA) and neuraminidase (NA) of CIV to the 2009 human pandemic virus results in a virus that replicates at high levels and causes significant lesions. This provides important insights into the role of evolution on viral emergence and on the role of HA and NA as determinants of pathogenicity.
Importance: Influenza A viruses (IAVs) have entered new host species in recent history, sometimes with devastating consequences. Canine influenza virus (CIV) H3N8 originated from a direct transfer of an equine influenza virus (EIV) in the early 2000s. We studied the infection patterns of IAVs that circulate in dogs or to which dogs are commonly exposed and showed that CIV emergence was likely caused by an adaptive driver, as evolutionarily distinct EIVs display distinct infection phenotypes. We also showed that many human viruses can infect dog tracheas and that reassortment with CIV results in viable viruses. Finally, we showed that the hemagglutinin and neuraminidase of CIV act as virulence factors. Our findings have significant implications because they show that dogs might act as "mixing vessels" in which novel viruses with pandemic potential could emerge and also provide experimental evidence supporting the role of viral evolution in influenza virus emergence.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
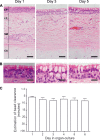

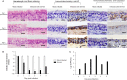



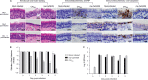


References
-
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. 2013. New World bats harbor diverse influenza A viruses. PLoS Pathog. 9:e1003657. 10.1371/journal.ppat.1003657 - DOI - PMC - PubMed
-
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368:1888–1897. 10.1056/NEJMoa1304459 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

