An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice
- PMID: 24899195
- PMCID: PMC4136293
- DOI: 10.1128/JVI.00327-14
An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice
Abstract
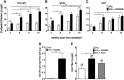
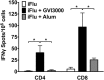
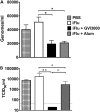
Neonatal immune responses to infection and vaccination are biased toward TH2 at the cost of proinflammatory TH1 responses needed to combat intracellular pathogens. However, upon appropriate stimulation, the neonatal immune system can induce adult-like TH1 responses. Here we report that a new class of vaccine adjuvant is especially well suited to enhance early life immunity. The GVI3000 adjuvant is a safe, nonpropagating, truncated derivative of Venezuelan equine encephalitis virus that targets dendritic cells (DCs) in the draining lymph node (DLN) and produces intracellular viral RNA without propagating to other cells. RNA synthesis strongly activates the innate immune response so that in adult animals, codelivery of soluble protein antigens induces robust humoral, cellular, and mucosal responses. The adjuvant properties of GVI3000 were tested in a neonatal BALB/c mouse model using inactivated influenza virus (iFlu). After a single immunization, mice immunized with iFlu with the GVI3000 adjuvant (GVI3000-adjuvanted iFlu) had significantly higher and sustained influenza virus-specific IgG antibodies, mainly IgG2a (TH1), compared to the mice immunized with antigen only. GVI3000 significantly increased antigen-specific CD4(+) and CD8(+) T cells, primed mucosal immune responses, and enhanced protection from lethal challenge. As seen in adult mice, the GVI3000 adjuvant increased the DC population in the DLNs, caused activation and maturation of DCs, and induced proinflammatory cytokines and chemokines in the DLNs soon after immunization, including gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), granulocyte colony-stimulating factor (G-CSF), and interleukin 6 (IL-6). In summary, the GVI3000 adjuvant induced an adult-like adjuvant effect with an influenza vaccine and has the potential to improve the immunogenicity and protective efficacy of new and existing neonatal vaccines.
Importance: The suboptimal immune responses in early life constitute a significant challenge for vaccine design. Here we report that a new class of adjuvant is safe and effective for early life immunization and demonstrate its ability to significantly improve the protective efficacy of an inactivated influenza virus vaccine in a neonatal mouse model. The GVI3000 adjuvant delivers a truncated, self-replicating viral RNA into dendritic cells in the draining lymph node. Intracellular RNA replication activates a strong innate immune response that significantly enhances adaptive antibody and cellular immune responses to codelivered antigens. A significant increase in protection results from a single immunization. Importantly, this adjuvant also primed a mucosal IgA response, which is likely to be critical for protection during many early life infections.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



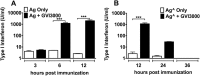
Similar articles
-
Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus.J Virol. 2010 Dec;84(24):12703-12. doi: 10.1128/JVI.01182-10. Epub 2010 Sep 29. J Virol. 2010. PMID: 20881038 Free PMC article.
-
Effects of different adjuvants in the context of intramuscular and intranasal routes on humoral and cellular immune responses induced by detergent-split A/H3N2 influenza vaccines in mice.Clin Vaccine Immunol. 2012 Feb;19(2):209-18. doi: 10.1128/CVI.05441-11. Epub 2011 Dec 21. Clin Vaccine Immunol. 2012. PMID: 22190392 Free PMC article.
-
DDO-adjuvanted influenza A virus nucleoprotein mRNA vaccine induces robust humoral and cellular type 1 immune responses and protects mice from challenge.mBio. 2025 Feb 5;16(2):e0358924. doi: 10.1128/mbio.03589-24. Epub 2024 Dec 18. mBio. 2025. PMID: 39692514 Free PMC article.
-
Mucosal immunization and adjuvants.Curr Top Microbiol Immunol. 2015;386:371-80. doi: 10.1007/82_2014_402. Curr Top Microbiol Immunol. 2015. PMID: 25015787 Free PMC article. Review.
-
Distinct and complementary roles of CD4 T cells in protective immunity to influenza virus.Curr Opin Immunol. 2018 Aug;53:13-21. doi: 10.1016/j.coi.2018.03.019. Epub 2018 Apr 2. Curr Opin Immunol. 2018. PMID: 29621639 Free PMC article. Review.
Cited by
-
Combination of a Sindbis-SARS-CoV-2 Spike Vaccine and αOX40 Antibody Elicits Protective Immunity Against SARS-CoV-2 Induced Disease and Potentiates Long-Term SARS-CoV-2-Specific Humoral and T-Cell Immunity.Front Immunol. 2021 Jul 29;12:719077. doi: 10.3389/fimmu.2021.719077. eCollection 2021. Front Immunol. 2021. PMID: 34394127 Free PMC article.
-
Short- and long-term protective efficacy against clade 2.3.4.4 H5N2 highly pathogenic avian influenza virus following prime-boost vaccination in turkeys.Vaccine. 2017 Oct 9;35(42):5637-5643. doi: 10.1016/j.vaccine.2017.08.059. Epub 2017 Sep 6. Vaccine. 2017. PMID: 28886943 Free PMC article.
-
Alphavirus Replicon Particle Vaccine Breaks B Cell Tolerance and Rapidly Induces IgG to Murine Hematolymphoid Tumor Associated Antigens.Front Immunol. 2022 May 24;13:865486. doi: 10.3389/fimmu.2022.865486. eCollection 2022. Front Immunol. 2022. PMID: 35686131 Free PMC article.
-
Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection.Vaccine. 2015 Sep 11;33(38):4892-900. doi: 10.1016/j.vaccine.2015.07.051. Epub 2015 Jul 29. Vaccine. 2015. PMID: 26232344 Free PMC article.
-
Inflammatory responses to influenza vaccination at the extremes of age.Immunology. 2017 Aug;151(4):451-463. doi: 10.1111/imm.12742. Epub 2017 May 16. Immunology. 2017. PMID: 28375554 Free PMC article.
References
-
- World Health Organization. 2014. World health statistics. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/112738/1/9789240692671_eng.pdf?...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

