Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial
- PMID: 24899268
- PMCID: PMC4106661
- DOI: 10.1001/jamasurg.2014.467
Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial
Abstract
Importance: Many questions remain unanswered about the role of bariatric surgery for people with type 2 diabetes mellitus (T2DM).
Objective: To determine feasibility of a randomized clinical trial (RCT) and compare initial outcomes of bariatric surgery and a structured weight loss program for treating T2DM in participants with grades I and II obesity.
Design, setting, and participants: A 12-month, 3-arm RCT at a single center including 69 participants aged 25 to 55 years with a body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 to 40 and T2DM.
Interventions: Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric banding (LAGB), and an intensive lifestyle weight loss intervention (LWLI).
Main outcomes and measures: Primary outcomes in the intention-to-treat cohort were feasibility and effectiveness measured by weight loss and improvements in glycemic control.
Results: Of 667 potential participants who underwent screening, 69 (10.3%) were randomized. Among the randomized participants, 30 (43%) had grade I obesity, and 56 (81%) were women. Mean (SD) age was 47.3 (6.4) years and hemoglobin A1c level, 7.9% (2.0%). After randomization, 7 participants (10%) refused to undergo their allocated intervention (3 RYGB, 1 LAGB, and 3 LWLI), and 1 RYGB participant was excluded for current smoking. Twenty participants underwent RYGB; 21, LAGB; and 20, LWLI, with 12-month retention rates of 90%, 86%, and 70%, respectively. In the intention-to-treat cohort with multiple imputation for missing data, RYGB participants had the greatest mean weight loss from baseline (27.0%; 95% CI, 30.8-23.3) compared with LAGB (17.3%; 95% CI, 21.1-13.5) and LWLI (10.2%; 95% CI, 14.8-5.61) (P < .001). Partial and complete remission of T2DM were 50% and 17%, respectively, in the RYGB group and 27% and 23%, respectively, in the LAGB group (P < .001 and P = .047 between groups for partial and complete remission), with no remission in the LWLI group. Significant reductions in use of antidiabetics occurred in both surgical groups. No deaths were noted. The 3 serious adverse events included 1 ulcer treated medically in the RYGB group and 2 rehospitalizations for dehydration in the LAGB group.
Conclusions and relevance: This study highlights several potential challenges to successful completion of a larger RCT for treatment of T2DM and obesity in patients with a body mass index of 30 to 40, including the difficulties associated with recruiting and randomizing patients to surgical vs nonsurgical interventions. Preliminary results show that RYGB was the most effective treatment, followed by LAGB for weight loss and T2DM outcomes at 1 year.
Trial registration: clinicaltrials.gov Identifier: NCT01047735.
Conflict of interest statement
Figures

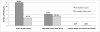
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

