A motif in LILRB2 critical for Angptl2 binding and activation
- PMID: 24899623
- PMCID: PMC4126332
- DOI: 10.1182/blood-2014-01-549162
A motif in LILRB2 critical for Angptl2 binding and activation
Abstract
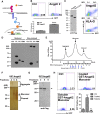
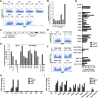
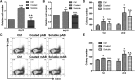
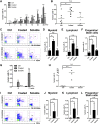
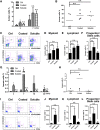
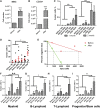
A better understanding of the interaction between extrinsic factors and surface receptors on stem cells will greatly benefit stem cell research and applications. Recently, we showed that several angiopoietin-like proteins (Angptls) bind and activate the immune inhibitory receptor human leukocyte immunoglobulin (Ig)-like receptor B2 (LILRB2) to support ex vivo expansion of hematopoietic stem cells (HSCs) and leukemia development. However, the molecular basis for the interaction between Angptls and LILRB2 was unclear. Here, we demonstrate that Angptl2 expressed in mammalian cells forms high-molecular-weight species and that ligand multimerization is required for activation of LILRB2 for downstream signaling. A novel motif in the first and fourth Ig domains of LILRB2 was identified that is necessary for the receptor to be bound and activated by Angptl2. The binding of Angptl2 to LILRB2 is more potent than and not completely overlapped with the binding of another ligand, HLA-G. Immobilized anti-LILRB2 antibodies induce a more potent activation of LILRB2 than Angptl2, and we developed a serum-free culture containing defined cytokines and immobilized anti-LILRB2 that supports a net expansion of repopulating human cord blood HSCs. Our elucidation of the mode of Angptl binding to LILRB2 enabled the development of a new approach for ex vivo expansion of human HSCs.
© 2014 by The American Society of Hematology.
Figures

Comment in
-
Unveiling Angptl2, a rising HSC expander.Blood. 2014 Aug 7;124(6):833-4. doi: 10.1182/blood-2014-06-581629. Blood. 2014. PMID: 25104862 Free PMC article.
References
-
- Brunstein CG, Wagner JE. Umbilical cord blood transplantation and banking. Annu Rev Med. 2006;57:403–417. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

