HSP70-binding protein HSPBP1 regulates chaperone expression at a posttranslational level and is essential for spermatogenesis
- PMID: 24899640
- PMCID: PMC4116300
- DOI: 10.1091/mbc.E14-02-0742
HSP70-binding protein HSPBP1 regulates chaperone expression at a posttranslational level and is essential for spermatogenesis
Abstract
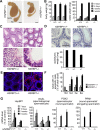
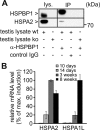
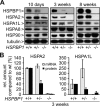
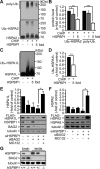
Molecular chaperones play key roles during growth, development, and stress survival. The ability to induce chaperone expression enables cells to cope with the accumulation of nonnative proteins under stress and complete developmental processes with an increased requirement for chaperone assistance. Here we generate and analyze transgenic mice that lack the cochaperone HSPBP1, a nucleotide-exchange factor of HSP70 proteins and inhibitor of chaperone-assisted protein degradation. Male HSPBP1(-/-) mice are sterile because of impaired meiosis and massive apoptosis of spermatocytes. HSPBP1 deficiency in testes strongly reduces the expression of the inducible, antiapoptotic HSP70 family members HSPA1L and HSPA2, the latter of which is essential for synaptonemal complex disassembly during meiosis. We demonstrate that HSPBP1 affects chaperone expression at a posttranslational level by inhibiting the ubiquitylation and proteasomal degradation of inducible HSP70 proteins. We further provide evidence that the cochaperone BAG2 contributes to HSP70 stabilization in tissues other than testes. Our findings reveal that chaperone expression is determined not only by regulated transcription, but also by controlled degradation, with degradation-inhibiting cochaperones exerting essential prosurvival functions.
© 2014 Rogon, Ulbricht, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Alberti S, Demand J, Esser C, Emmerich N, Schild H, Höhfeld J. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem. 2002;277:45920–45927. - PubMed
-
- Allen JW, Dix DJ, Collins BW, Merrick BA, He C, Selkirk JK, Poorman-Allen P, Dresser ME, Eddy EM. HSP70–2 is part of the synaptonemal complex in mouse and hamster spermatocytes. Chromosoma. 1996;104:414–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

