How local excitation-inhibition ratio impacts the whole brain dynamics
- PMID: 24899711
- PMCID: PMC4044249
- DOI: 10.1523/JNEUROSCI.5068-13.2014
How local excitation-inhibition ratio impacts the whole brain dynamics
Abstract
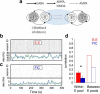
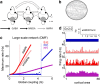
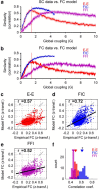
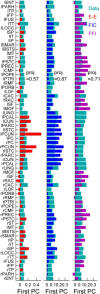
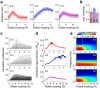
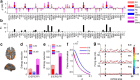
The spontaneous activity of the brain shows different features at different scales. On one hand, neuroimaging studies show that long-range correlations are highly structured in spatiotemporal patterns, known as resting-state networks, on the other hand, neurophysiological reports show that short-range correlations between neighboring neurons are low, despite a large amount of shared presynaptic inputs. Different dynamical mechanisms of local decorrelation have been proposed, among which is feedback inhibition. Here, we investigated the effect of locally regulating the feedback inhibition on the global dynamics of a large-scale brain model, in which the long-range connections are given by diffusion imaging data of human subjects. We used simulations and analytical methods to show that locally constraining the feedback inhibition to compensate for the excess of long-range excitatory connectivity, to preserve the asynchronous state, crucially changes the characteristics of the emergent resting and evoked activity. First, it significantly improves the model's prediction of the empirical human functional connectivity. Second, relaxing this constraint leads to an unrealistic network evoked activity, with systematic coactivation of cortical areas which are components of the default-mode network, whereas regulation of feedback inhibition prevents this. Finally, information theoretic analysis shows that regulation of the local feedback inhibition increases both the entropy and the Fisher information of the network evoked responses. Hence, it enhances the information capacity and the discrimination accuracy of the global network. In conclusion, the local excitation-inhibition ratio impacts the structure of the spontaneous activity and the information transmission at the large-scale brain level.
Keywords: anatomical connectivity; functional connectivity; large-scale brain model; local feedback inhibition; resting-state activity.
Copyright © 2014 the authors 0270-6474/14/347886-13$15.00/0.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
