The substantia nigra conveys target-dependent excitatory and inhibitory outputs from the basal ganglia to the thalamus
- PMID: 24899724
- PMCID: PMC4044258
- DOI: 10.1523/JNEUROSCI.0236-14.2014
The substantia nigra conveys target-dependent excitatory and inhibitory outputs from the basal ganglia to the thalamus
Abstract
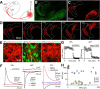
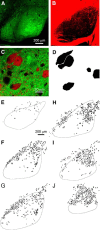
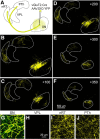
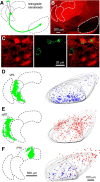
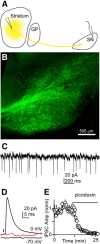
The basal ganglia (BG), which influence cortical activity via the thalamus, play a major role in motor activity, learning and memory, sensory processing, and many aspects of behavior. The substantia nigra (SN) consists of GABAergic neurons of the pars reticulata that inhibit thalamic neurons and provide the primary output of the BG, and dopaminergic neurons of the pars compacta that modulate thalamic excitability. Little is known about the functional properties of the SN→thalamus synapses, and anatomical characterization has been controversial. Here we use a combination of anatomical, electrophysiological, genetic, and optogenetic approaches to re-examine these synaptic connections in mice. We find that neurons in the SN inhibit neurons in the ventroposterolateral nucleus of the thalamus via GABAergic synapses, excite neurons in the thalamic nucleus reticularis, and both excite and inhibit neurons within the posterior nucleus group. Glutamatergic SN neurons express the vesicular glutamate receptor transporter vGluT2 and receive inhibitory synapses from striatal neurons, and many also express tyrosine hydroxylase, a marker of dopaminergic neurons. Thus, in addition to providing inhibitory outputs, which is consistent with the canonical circuit, the SN provides glutamatergic outputs that differentially target thalamic nuclei. This suggests that an increase in the activity of glutamatergic neurons in the SN allows the BG to directly excite neurons in specific thalamic nuclei. Elucidating an excitatory connection between the BG and the thalamus provides new insights into how the BG regulate thalamic activity, and has important implications for understanding BG function in health and disease.
Keywords: circuitry; substantia nigra; vGluT2.
Copyright © 2014 the authors 0270-6474/14/348032-11$15.00/0.
Figures

References
-
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. - DOI - PMC - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. In: Uylings HBM, editor. The prefrontal cortex: its structure, function and pathology; Progress in Brain Research. New York: Elsevier; 1991. pp. 119–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
