Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist
- PMID: 24900187
- PMCID: PMC4007689
- DOI: 10.1021/ml1000307
Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist
Abstract
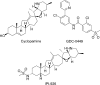
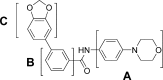
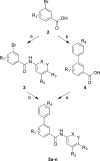
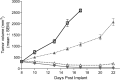
The blockade of aberrant hedgehog (Hh) signaling has shown promise for therapeutic intervention in cancer. A cell-based phenotypic high-throughput screen was performed, and the lead structure (1) was identified as an inhibitor of the Hh pathway via antagonism of the Smoothened receptor (Smo). Structure-activity relationship studies led to the discovery of a potent and specific Smoothened antagonist N-(6-((2S,6R)-2,6-dimethylmorpholino)pyridin-3-yl)-2-methyl-4'-(trifluoromethoxy)biphenyl-3-carboxamide (5m, NVP-LDE225), which is currently in clinical development.
Keywords: Hedgehog signaling pathway; Smoothened; medulloblastoma.
Figures
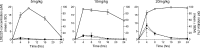
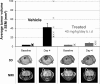
References
-
- Stone D. M.; Hynes M.; Armanini M.; Swanson T. A.; Gu Q.; Johnson R. L.; Scott M. P.; Pennica D.; Goddard A.; Phillips H.; Noll M.; Hooper J. E.; de Sauvage F.; Rosenthal A. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 1996, 384, 129–134. - PubMed
-
- McMahon A. P.; Ingham P. W.; Tabin C. J. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol. 2003, 53, 1–114. - PubMed
-
- Ingham P. W.; McMahon A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001, 15, 3059–3087. - PubMed
-
- di Magliano M. P.; Hebrok M. Hedgehog signaling in cancer formation and maintenance. Nat. Rev. Cancer 2003, 3, 903–911. - PubMed
-
- Rubin L. L.; de Sauvage F. J. Targeting the hedgehog pathway in cancer. Nat. Rev. Drug Discovery 2006, 5, 1026–1033. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

