[Au2(phen(2Me))2(μ-O)2](PF6)2, a Novel Dinuclear Gold(III) Complex Showing Excellent Antiproliferative Properties
- PMID: 24900215
- PMCID: PMC4007953
- DOI: 10.1021/ml100097f
[Au2(phen(2Me))2(μ-O)2](PF6)2, a Novel Dinuclear Gold(III) Complex Showing Excellent Antiproliferative Properties
Abstract
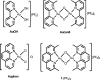
A novel dioxo-bridged dinuclear gold(III) complex with two 2,9-dimethylphenanthroline ligands was synthesized and thoroughly characterized. Its crystal structure was solved, and its solution behavior assessed. Remarkably, this compound revealed excellent antiproliferative properties in vitro against a wide panel of 36 cancer cell lines, combining a high cytotoxic potency to pronounced tumor selectivity. Very likely, these properties arise from an innovative mode of action (possibly involving histone deacetylase inhibition), as suggested by COMPARE analysis. In turn, electrospray ionization-mass spectrometry studies provided valuable insight into its molecular mechanisms of activation and of interaction with protein targets. Gold(III) reduction, dioxo bridge disruption, coordinative gold(I) binding to the protein, and concomitant release of the phenanthroline ligand were proposed to occur upon interaction with superoxide dismutase, used here as a model protein. Because of the reported results, this new gold(III) compound qualifies itself as an optimal candidate for further pharmacological testing.
Keywords: COMPARE analysis; Cytotoxic gold compounds; anticancer agents; protein targets.
Figures


References
-
- Rosenberg B.; Vancamp L.; Krigas T. Nature 1965, 205, 698. - PubMed
-
- Wang D.; Lippard S. J. Nature Rev. Drug Discovery 2005, 4, 307–320. - PubMed
-
- Cohen S. M.; Lippard S. J. Cisplatin: From DNA damage to cancer chemotherapy. Prog. Nucleic Acid Res. Mol. Biol. 2001, 67, 93–130. - PubMed
-
- Barnes K. R.; Lippard S. J.. Cisplatin and related anticancer drugs: recent advances and insights. In Metal Ions in Biological Systems; Sigel, A. and Sigel, H., Eds; Marcel Dekker: New York, 2004; Vol. 42, pp 143−177. - PubMed
-
- Hartinger C. G.; Dyson P. J. Chem. Soc. Rev. 2009, 38, 391. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

