Discovery of BIIB042, a Potent, Selective, and Orally Bioavailable γ-Secretase Modulator
- PMID: 24900267
- PMCID: PMC4018072
- DOI: 10.1021/ml200175q
Discovery of BIIB042, a Potent, Selective, and Orally Bioavailable γ-Secretase Modulator
Abstract
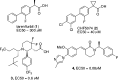
We have investigated a novel series of acid-derived γ-secretase modulators as a potential treatment of Alzheimer's disease. Optimization based on cellular potency and brain pharmacodynamics after oral dosing led to the discovery of 10a (BIIB042). Compound 10a is a potent γ-secretase modulator, which lowered Aβ42, increased Aβ38, but had little to no effect on Aβ40 levels both in vitro and in vivo. In addition, compound 10a did not affect Notch signaling in our in vitro assessment. Compound 10a demonstrated excellent pharmacokinetic parameters in multiple species. Oral administration of 10a significantly reduced brain Aβ42 levels in CF-1 mice and Fischer rats, as well as plasma Aβ42 levels in cynomolgus monkeys. Compound 10a was selected as a candidate for preclinical safety evaluation.
Keywords: Alzheimer's; GSM; Mannich reaction; Secretase; amyloid.
Figures


References
-
- Citron M. Alzheimer's disease: Strategies for disease modification. Nature Rev. Drug Discovery 2010, 9, 387–398. - PubMed
-
- Hardy J.; Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. - PubMed
-
- Tanzi R. E.; Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: A genetic perspective. Cell 2005, 120, 545–555. - PubMed
-
- Walsh D. M.; Selkoe D. J. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron 2004, 44, 181–193. - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information

