Discovery of a Selective S1P1 Receptor Agonist Efficacious at Low Oral Dose and Devoid of Effects on Heart Rate
- PMID: 24900328
- PMCID: PMC4018134
- DOI: 10.1021/ml2000214
Discovery of a Selective S1P1 Receptor Agonist Efficacious at Low Oral Dose and Devoid of Effects on Heart Rate
Abstract
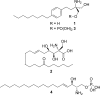
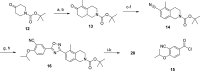
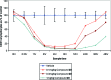
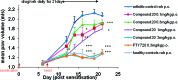
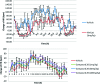
Gilenya (fingolimod, FTY720) was recently approved by the U.S. FDA for the treatment of patients with remitting relapsing multiple sclerosis (RRMS). It is a potent agonist of four of the five sphingosine 1-phosphate (S1P) G-protein-coupled receptors (S1P1 and S1P3-5). It has been postulated that fingolimod's efficacy is due to S1P1 agonism, while its cardiovascular side effects (transient bradycardia and hypertension) are due to S1P3 agonism. We have discovered a series of selective S1P1 agonists, which includes 3-[6-(5-{3-cyano-4-[(1-methylethyl)oxy]phenyl}-1,2,4-oxadiazol-3-yl)-5-methyl-3,4-dihydro-2(1H)-isoquinolinyl]propanoate, 20, a potent, S1P3-sparing, orally active S1P1 agonist. Compound 20 is as efficacious as fingolimod in a collagen-induced arthritis model and shows excellent pharmacokinetic properties preclinically. Importantly, the selectivity of 20 against S1P3 is responsible for an absence of cardiovascular signal in telemetered rats, even at high dose levels.
Keywords: Fingolimod; S1P; agonism; bradycardia; lymphopenia; multiple sclerosis.
Figures
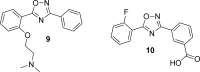

References
-
- Martini S.; Peters H.; Böhler T.; Budde K. Current Perspectives on FTY720. Expert Opin. Invest. Drugs 2007, 16, 505–518. - PubMed
-
- Budde K.; Schütz M.; Glander P.; Peters H.; Waiser J.; Liefeldt L.; Neumayer H-H; Böhler T. FTY720 (fingolimod) in renal transplantation. Clin. Transplant. 2006, 20Suppl. 1717–24. - PubMed
-
- Kappos L.; Antel J.; Comi G.; Montalban X.; O'Connor P.; Polman C. H.; Haas T.; Korn A. A.; Karlsson G.; Radue E. W. Oral Fingolimod (FTY720) for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 355, 1124–1139. - PubMed
-
- Billich A.; Bornancin F.; Dévay P.; Mechtcheriakova D.; Urtz N.; Baumruker T. Phosphorylation of the Immunomodulatory Drug FTY720 by Sphingosine Kinases. J. Biol. Chem. 2003, 278, 47408–47415. - PubMed
-
- Albert R.; Hinterding K.; Brinkmann V.; Guerini D.; Müller-Hartwieg C.; Knecht H.; Simeon C.; Streiff M.; Wagner T.; Welzenbach K.; Zécri F.; Zollinger M.; Cooke N.; Francotte E. Novel Immunomodulator FTY720 Is Phosphorylated in Rats and Humans to Form a Single Stereoisomer. Identification, Chemical Proof, and Biological Characterisation of the Biologically Active Species and Its Enantiomer. J. Med. Chem. 2005, 48, 5373–5377. - PubMed
LinkOut - more resources
Full Text Sources

