Discovery of CX-6258. A Potent, Selective, and Orally Efficacious pan-Pim Kinases Inhibitor
- PMID: 24900437
- PMCID: PMC4025662
- DOI: 10.1021/ml200259q
Discovery of CX-6258. A Potent, Selective, and Orally Efficacious pan-Pim Kinases Inhibitor
Abstract
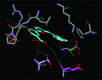
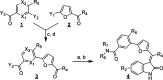
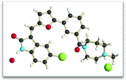
Structure-activity relationship analysis in a series of 3-(5-((2-oxoindolin-3-ylidene)methyl)furan-2-yl)amides identified compound 13, a pan-Pim kinases inhibitor with excellent biochemical potency and kinase selectivity. Compound 13 exhibited in vitro synergy with chemotherapeutics and robust in vivo efficacy in two Pim kinases driven tumor models.
Keywords: CX-6258; MV-4-11; PC3; Pim-1; Pim-2; Pim-3.
Figures


References
-
- Nawijn M. C.; Alendar A.; Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat. Rev. Cancer 2010, 11, 23–34. - PubMed
-
- Eichmann A.; Yuan L.; Breant C.; Alitalo K.; Koskinen P. J. Developmental expression of pim kinases suggests functions also outside of the hematopoietic system. Oncogene 2000, 1991215–24. - PubMed
-
- Allen J. D.; Verhoeven E.; Domen J.; van der Valk M.; Berns A. Pim-2 transgene induces lymphoid tumors, exhibiting potent synergy with c-myc. Oncogene 1997, 15101133–41. - PubMed
-
- Yip-Schneider M. T.; Horie M.; Broxmeyer H. E. Transcriptional induction of pim-1 protein kinase gene expression by interferon gamma and posttranscriptional effects on costimulation with steel factor. Blood 1995, 85123494–502. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

