β-Lactones Inhibit N-acylethanolamine Acid Amidase by S-Acylation of the Catalytic N-Terminal Cysteine
- PMID: 24900487
- PMCID: PMC4025845
- DOI: 10.1021/ml300056y
β-Lactones Inhibit N-acylethanolamine Acid Amidase by S-Acylation of the Catalytic N-Terminal Cysteine
Abstract
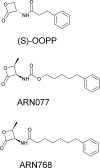
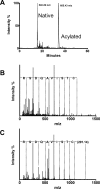
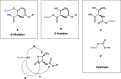
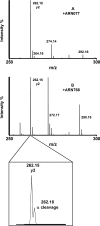
The cysteine amidase N-acylethanolamine acid amidase (NAAA) is a member of the N-terminal nucleophile class of enzymes and a potential target for anti-inflammatory drugs. We investigated the mechanism of inhibition of human NAAA by substituted β-lactones. We characterized pharmacologically a representative member of this class, ARN077, and showed, using high-resolution liquid chromatography-tandem mass spectrometry, that this compound forms a thioester bond with the N-terminal catalytic cysteine in human NAAA.
Keywords: NAAA; covalent inhibitors; cysteine amidase; high resolution mass spectrometry; proteomics.
Figures

References
-
- Devane W. A.; Hanus L.; Breuer A.; Pertwee R. G.; Stevenson L. A.; Griffin G.; Gibson D.; Mandelbaum A.; Etinger A.; Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. - PubMed
-
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. - PubMed
-
- Kathuria S.; Gaetani S.; Fegley D.; Valino F.; Duranti A.; Tontini A.; Mor M.; Tarzia G.; La Rana G.; Calignano A.; Giustino A.; Tattoli M.; Palmery M.; Cuomo V.; Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. - PubMed
-
- Gobbi G.; Bambico F. R.; Mangieri R.; Bortolato M.; Campolongo P.; Solinas M.; Cassano T.; Morgese M. G.; Debonnel G.; Duranti A.; Tontini A.; Tarzia G.; Mor M.; Trezza V.; Goldberg S. R.; Cuomo V.; Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 18620–18625. - PMC - PubMed
-
- Bortolato M.; Mangieri R. A.; Fu J.; Kim J. H.; Arguello O.; Duranti A.; Tontini A.; Mor M.; Tarzia G.; Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatry 2007, 62, 1103–1110. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

