Target-Activated Prodrugs (TAPs) for the Autoregulated Inhibition of MMP12
- PMID: 24900526
- PMCID: PMC4025753
- DOI: 10.1021/ml3001193
Target-Activated Prodrugs (TAPs) for the Autoregulated Inhibition of MMP12
Abstract
We describe a prodrug concept in which the target enzyme MMP12 produces its own inhibitor in a two-step activation procedure. By using an MMP12-specific peptide sequence and a known sulfonamide drug integrated in the backbone, the active inhibitor is released upon enzyme cleavage. In in vitro experiments, we present proof of concept that the activation proceeds with useful kinetics. The approach is highly selective over the closely related MMP8. If applied in vivo in the future, these prodrugs might release the active entity in a highly specific manner only at such sites where enzyme activity resides.
Keywords: MMP; inflammation; inhibitors; prodrug; proteases.
Figures
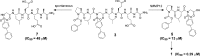

Similar articles
-
Increasing oral absorption of polar neuraminidase inhibitors: a prodrug transporter approach applied to oseltamivir analogue.Mol Pharm. 2013 Feb 4;10(2):512-22. doi: 10.1021/mp300564v. Epub 2013 Jan 4. Mol Pharm. 2013. PMID: 23244438 Free PMC article.
-
Targeting an MMP-9-activated prodrug to multiple myeloma-diseased bone marrow: a proof of principle in the 5T33MM mouse model.Leukemia. 2005 Sep;19(9):1628-33. doi: 10.1038/sj.leu.2403866. Leukemia. 2005. PMID: 16015389
-
Evaluation of a GSH-targeting prodrug via a sulfonamide-induced "integrative" platform for selective cancer therapy.Analyst. 2020 Jul 13;145(14):4901-4905. doi: 10.1039/d0an00627k. Analyst. 2020. PMID: 32478783
-
Activation of oxazaphosphorines by cytochrome P450: application to gene-directed enzyme prodrug therapy for cancer.Toxicol In Vitro. 2006 Mar;20(2):176-86. doi: 10.1016/j.tiv.2005.06.046. Epub 2005 Nov 15. Toxicol In Vitro. 2006. PMID: 16293390 Review.
-
Targeted prodrug approaches for hormone refractory prostate cancer.Med Res Rev. 2015 May;35(3):554-85. doi: 10.1002/med.21333. Epub 2014 Dec 22. Med Res Rev. 2015. PMID: 25529338 Review.
Cited by
-
Genetic deletion of MMP12 ameliorates cardiometabolic disease by improving insulin sensitivity, systemic inflammation, and atherosclerotic features in mice.Cardiovasc Diabetol. 2023 Nov 28;22(1):327. doi: 10.1186/s12933-023-02064-3. Cardiovasc Diabetol. 2023. PMID: 38017481 Free PMC article.
-
Controlled release strategy of paclitaxel by conjugating to matrix metalloproteinases-2 sensitive peptide.Oncotarget. 2016 Aug 9;7(32):52230-52238. doi: 10.18632/oncotarget.10735. Oncotarget. 2016. PMID: 27447567 Free PMC article.
References
-
- Turk B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discovery 2006, 59785–799. - PubMed
-
- Belaaouaj A.; Shipley J. M.; Kobayashi D. K.; Zimonjic D. B.; Popescu N.; Silverman G. A.; Shapiro S. D. Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J. Biol. Chem. 1995, 2702414568–14575. - PubMed
-
- Lagente V. L.; Le Quement C.; Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin. Ther. Targets 2009, 133287–295. - PubMed
-
- Uttamchandani M. W.; Wang J.; Li J.; Hu M.; Sun H.; Chen K. Y.; Liu K.; Yao S. Q. Inhibitor fingerprinting of matrix metalloproteases using a combinatorial peptide hydroxamate library. J. Am. Chem. Soc. 2007, 129257848–7858. - PubMed
-
- Hu J.; Van den Steen P. E.; Sang Q. X.; Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discovery 2007, 66480–498. - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

