Azepines and piperidines with dual norepinephrine dopamine uptake inhibition and antidepressant activity
- PMID: 24900562
- PMCID: PMC4027539
- DOI: 10.1021/ml300262e
Azepines and piperidines with dual norepinephrine dopamine uptake inhibition and antidepressant activity
Abstract
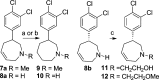
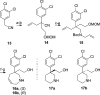
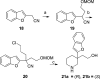
Herein, we describe the discovery of inhibitors of norepinephrine (NET) and dopamine (DAT) transporters with reduced activity relative to serotonin transporters (SERT). Two compounds, 8b and 21a, along with nomifensine were tested in a rodent receptor occupancy study and demonstrated dose-dependent displacement of radiolabeled NET and DAT ligands. These compounds were efficacious in a rat forced swim assay (model of depression) and also had activity in rat spontaneous locomotion assay.
Keywords: antidepressant; dopamine; norepinephrine; uptake inhibitors.
Figures



References
-
- Nemeroff C. B. Prevalence and Management of Treatment-Resistant Depression. J. Clin. Psychiatry 2007, 68Suppl. 817–25. - PubMed
-
- Papakostas G. Limitations of Contemporary Antidepressants: Tolerability. J. Clin. Psychiatry 2007, 68, 11–17. - PubMed
-
- Liu S.; Molino B. F. Recent Developments in Monoamine Reuptake Inhibitors. Annu. Rep. Med. Chem. 2007, 42, 13–26.
-
- Wong D. T.; Perry K. W.; Bymaster F. P. The Discovery of Fluoxetine Hydrochloride (Prozac). Nat. Rev. Drug Discovery 2005, 49764–774. - PubMed
-
- Schatzberg A. F. Efficacy and Tolerability of Duloxetine, a Novel Dual Uptake Inhibitor, in the Treatment of Major Depressive Disorder. J. Clin. Psychiatry 2003, 64Suppl. 1330–37. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

