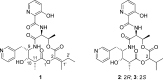Synthesis and antimycobacterial activity of 2,1'-dihydropyridomycins
- PMID: 24900646
- PMCID: PMC4027526
- DOI: 10.1021/ml300385q
Synthesis and antimycobacterial activity of 2,1'-dihydropyridomycins
Abstract
Dihydropyridomycins 2 and 3, which lack the characteristic enol ester moiety of the potent antimycobacterial natural product pyridomycin (1), have been prepared from l-Thr, R- and S-hydroxy isovaleric acid, and 3-pyridinecarboxaldehyde. The 2R isomer 2 shows only 4-fold lower anti-Mtb activity than 1, indicating that the enol ester moiety in the natural product is not critical for its biological activity. This finding establishes 2 as a potent and more practical lead for anti-TB drug discovery.
Keywords: InhA; natural products; pyridomycin; total synthesis; tuberculosis.
Figures
References
-
- Glaziou P.; Floyd K.; Raviglione M. Global Burden and Epidemiology of Tuberculosis. Clin. Chest Med. 2009, 30, 621–636. - PubMed
-
- Cegielski J. P. Extensively Drug-Resistant Tuberculosis: “There Must Be Some Kind of Way Out of Here”. Clin. Infect. Dis. 2010, 50, 195–200. - PubMed
-
- Maeda K.; Kosaka H.; Okami Y.; Umezawa H. A New Antibiotic, Pyridomycin. J. Antibiot., Ser. A 1953, 6, 140. - PubMed
-
- The structure and absolute configuration of 1 were first reported in 1967:Koyama G.; Iitaka Y.; Maeda K.; Umezawa H. Structure of Pyridomycin. Tetrahedron Lett. 1967, 37, 3587–3590.Note that the structural drawing of 1 in this paper depicts a R configuration for both C9 and C10. In contrast, the drawing of the X-ray structure of the molecule in Figure 1 of the same paper shows these chiral centers to be present in a S,S configuration. As in the new analogues 2 and 3 that are reported here for the first time, C9 and C10 were both S-configured in the synthetic pyridomycin reported by Kinoshita et al. (ref (8)); the synthetic material was found to be identical spectroscopically with natural pyridomycin. - PubMed
-
- Kuroya M.; Takahashi B.; Hinuma Y.; Yashima T.; Watanabe K.; Hamada S. An Antituberculous Antibiotic from Streptomyces sp. Studies on the Streptomyces Antibiotics. XXXII. J. Antibiot., Ser. A 1954, 7, 58–59. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information