Enhancing a CH-π Interaction to Increase the Affinity for 5-HT1A Receptors
- PMID: 24900840
- PMCID: PMC4027751
- DOI: 10.1021/ml4004843
Enhancing a CH-π Interaction to Increase the Affinity for 5-HT1A Receptors
Abstract
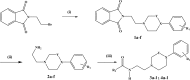
An electrostatic interaction related to a favorable position of the distal phenyl ring and a phenylalanine residue in the binding pocket would explain the higher 5-HT1A affinity of a 4-phenyl-1,2,3,6-tetrahydropyridine (THP) analogue compared to the corresponding 4-phenylpiperazine analogue. To explore a possible reinforcement of this interaction to increase the affinity for 5-HT1A receptors, different 4-substituted-phenyl analogues were synthesized and tested. The most important increase of affinity is obtained with two electron-donating methyl groups in positions 3 and 5.
Keywords: CH−π interaction; arylpiperazine; carboxamide; docking; electron-donating; quinoxaline.
Figures

References
-
- Graulich A.; Léonard M.; Résimont M.; Huang X.; Roth B. L.; Liégeois J.-F. Chemical modifications on 4-arylpiperazine-ethyl carboxamide derivatives differentially modulate affinity for 5-HT1A, D4.2, and α2A receptors: synthesis and in vitro radioligand binding studies. Aust. J. Chem. 2010, 63, 56–67.
-
- Dilly S.; Graulich A.; Liégeois J.-F. Molecular modeling study of 4-phenylpiperazine and 4-phenyl-1,2,3,6-tetrahydropyridine derivatives: a new step towards the design of high-affinity 5-HT1A ligands. Bioorg. Med. Chem. Lett. 2010, 20, 1118–23. - PubMed
-
- Cockroft S. L.; Perkins J.; Zonta C.; Adams H.; Spey S. E.; Low C. M.; Vinter J. G.; Lawson K. R.; Urch C. J.; Hunter C. A. Substituent effects on aromatic stacking interactions. Org. Biomol. Chem. 2007, 5, 1062–80. - PubMed
-
- Gallardo-Godoy A.; Fierro A.; McLean T. H.; Castillo M.; Cassels B. K.; Reyes-Parada M.; Nichols D. E. Sulfur-substituted alpha-alkyl phenethylamines as selective and reversible MAO-A inhibitors: biological activities, CoMFA analysis, and active site modeling. J. Med. Chem. 2005, 48, 2407–19. - PubMed
-
- Pettersson F.; Svensson P.; Waters S.; Waters N.; Sonesson C. Synthesis, pharmacological evaluation and QSAR modeling of mono-substituted 4-phenylpiperidines and 4-phenylpiperazines. Eur. J. Med. Chem. 2013, 62, 241–55. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

