3-Amido-3-aryl-piperidines: A Novel Class of Potent, Selective, and Orally Active GlyT1 Inhibitors
- PMID: 24900853
- PMCID: PMC4027785
- DOI: 10.1021/ml500005m
3-Amido-3-aryl-piperidines: A Novel Class of Potent, Selective, and Orally Active GlyT1 Inhibitors
Abstract
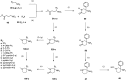
3-Amido-3-aryl-piperidines were discovered as a novel structural class of GlyT1 inhibitors. The structure-activity relationship, which was developed, led to the identification of highly potent compounds exhibiting excellent selectivity against the GlyT2 isoform, drug-like properties, and in vivo activity after oral administration.
Keywords: 3-amido-3-aryl-piperidines; GlyT1 inhibitor; SAR; Schizophrenia.
Figures





References
-
- Chue P. Glycine reuptake inhibition as a new therapeutic approach in schizophrenia: focus on the glycine transporter 1 (GlyT1). Curr. Pharm. Des. 2013, 19, 1311–1320. - PubMed
-
- Atkinson B. N.; Bell S. C.; De Vivo M.; Kowalski L. R.; Lechner S. M.; Ognyanov V. I.; Tham C.-S.; Tsai C.; Jia J.; Ashton D.; Klitenick M. A. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol. Pharmacol. 2001, 60, 1414–1420. - PubMed
-
- Brown A.; Carlyle I.; Clark J.; Hamilton W.; Gibson S.; McGarry G.; McEachen S.; Rae D.; Thorn S.; Walker G. Discovery and SAR of Org 24598: a selective glycine uptake inhibitor. Bioorg. Med. Chem. Lett. 2001, 11, 2007–2009. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

