Implementation study of patient-ready syringes containing 25 mg/mL methotrexate solution for use in treating ectopic pregnancy
- PMID: 24900977
- PMCID: PMC4037123
- DOI: 10.1155/2014/689308
Implementation study of patient-ready syringes containing 25 mg/mL methotrexate solution for use in treating ectopic pregnancy
Abstract
Background: Ectopic pregnancy (EP) is a significant cause of morbidity and mortality during the first trimester of pregnancy. Small unruptured tubal pregnancies can be treated medically with a single dose of methotrexate (MTX).
Objective: The aim of this study was to evaluate the stability of a 25 mg/mL solution of MTX to devise a secure delivery circuit for the preparation and use of this medication in the management of EP.
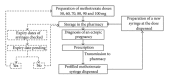
Method: MTX solutions were packaged in polypropylene syringes, stored over an 84-day period, and protected from light either at +2 to +8°C or at 23°C. We assessed the physical and chemical stability of the solutions at various time points over the storage period. A pharmaceutical delivery circuit was implemented that involved the batch preparation of MTX syringes.
Results: We show that 25 mg/mL MTX solutions remain stable over an 84-day period under the storage conditions tested. Standard doses were prepared, ranging from 50 mg to 100 mg. The results of this study suggest that MTX syringes can be prepared in advance by the pharmacy, ready to be dispensed at any time that a diagnosis of EP is made.
Conclusion: The high stability of a 25 mg/mL MTX solution in polypropylene syringes makes it possible to implement a flexible and cost-effective delivery circuit for ready-to-use preparations of this drug, providing 24-hour access and preventing treatment delays.
References
-
- Farquhar CM. Ectopic pregnancy. The Lancet. 2005;366(9485):583–591. - PubMed
-
- Fernandez H, Capmas P, Lucot JP, Resch B, Panel P, Bouyer J. GROG. Fertility after ectopic pregnancy: the DEMETER randomized trial. Human Reproduction. 2013;28(5):1247–1253. - PubMed
-
- Tanaka T, Hayashi H, Kutsuzawa T, Fujimoto S, Ichinoe K. Treatment of interstitial ectopic pregnancy with methotrexate: report of a successful case. Fertility and Sterility. 1982;37(6):851–852. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical