Retinoblastoma protein (RB) interacts with E2F3 to control terminal differentiation of Sertoli cells
- PMID: 24901045
- PMCID: PMC4611710
- DOI: 10.1038/cddis.2014.232
Retinoblastoma protein (RB) interacts with E2F3 to control terminal differentiation of Sertoli cells
Abstract
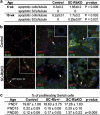
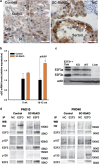
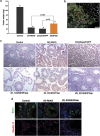
The retinoblastoma protein (RB) is essential for normal cell cycle control. RB function depends, at least in part, on interactions with the E2F family of DNA-binding transcription factors (E2Fs). To study the role of RB in the adult testis, a Sertoli cell (SC)-specific Rb knockout mouse line (SC-RbKO) was generated using the Cre/loxP recombination system. SC-RbKO mice exhibited an age-dependent testicular atrophy, impaired fertility, severe SC dysfunction, and spermatogenic defects. Removal of Rb in SC induced aberrant SC cycling, dedifferentiation, and apoptosis. Here we show that E2F3 is the only E2F expressed in mouse SCs and that RB interacts with E2F3 during mouse testicular development. In the absence of RB, the other retinoblastoma family members p107 and p130 began interacting with E2F3 in the adult testes. In vivo silencing of E2F3 partially restored the SC maturation and survival as well as spermatogenesis in the SC-RbKO mice. These results point to RB as a key regulator of SC function in adult mice and that the RB/E2F3 pathway directs SC maturation, cell cycle quiescence, and RB protects SC from apoptosis.
Figures



References
-
- 1Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer 2002; 2: 910–917. - PubMed
-
- 3Yan W, Kero J, Suominen J, Toppari J. Differential expression and regulation of the retinoblastoma family of proteins during testicular development and spermatogenesis: roles in the control of germ cell proliferation, differentiation and apoptosis. Oncogene 2001; 20: 1343–1356. - PubMed
-
- 4Yan W, West A, Toppari J, Lahdetie J. Stage-specific expression and phosphorylation of retinoblastoma protein (pRb) in the rat seminiferous epithelium. Mol Cell Endocrinol 1997; 132: 137–148. - PubMed
-
- 5Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML et al. Requirement for a functional Rb-1 gene in murine development. Nature 1992; 359: 328–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

