Identification of aurora kinase A as an unfavorable prognostic factor and potential treatment target for metastatic gastrointestinal stromal tumors
- PMID: 24901229
- PMCID: PMC4147307
- DOI: 10.18632/oncotarget.1705
Identification of aurora kinase A as an unfavorable prognostic factor and potential treatment target for metastatic gastrointestinal stromal tumors
Abstract
Although imatinib mesylate (IM) has revolutionized the management of gastrointestinal stromal tumors (GISTs), drug resistance remains a challenge. Previous studies have shown that the expression of aurora kinase A (AURKA) predicts recurrence in patients with primary, surgically resected GISTs. The current study aimed to evaluate the significance of AURKA expression as an unfavorable prognostic marker for advanced GISTs, and provide evidence that AURKA could be a potential therapeutic target in GISTs. The prognostic significance of the expression of AURKA, along with other clinicopathological factors, was analyzed in a cohort of 99 IM-treated patients with advanced GISTs. The potential use of an inhibitor of AURKA as a therapeutic agent against GISTs was also tested in GIST cell lines. Among 99 enrolled patients, poor performance status, large tumor size, drug response, and AURKA overexpression were independent prognostic factors for poor progression-free survival (PFS). For overall survival (OS), only large tumor size and AURKA overexpression were identified as independent unfavorable factors. In an in vitro study, MLN8237, an AURKA inhibitor, inhibited growth of both IM-sensitive and IM-resistant GIST cells in a concentration-dependent manner, and exhibited synergistic cytotoxicity with IM in GIST cells. The inhibitory effect of MLN8237 in GIST cells could be attributed to the induction of G2/M arrest, apoptosis, and senescence. Our study shows that AURKA expression independently predicted poor PFS and OS in patients with advanced GISTs who were treated with IM. An AURKA inhibitor may have potential as a therapeutic agent for both IM-sensitive and IM-resistant GISTs.
Conflict of interest statement
None declared.
Figures
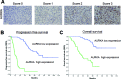

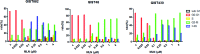




References
-
- Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100(1):162–168. - PubMed
-
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–1478. - PubMed
-
- Reith JD, Goldblum JR, Lyles RH, Weiss SW. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13(5):577–585. - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad TG, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

