Interactome profile of the host cellular proteins and the nonstructural protein 2 of porcine reproductive and respiratory syndrome virus
- PMID: 24901321
- PMCID: PMC4047090
- DOI: 10.1371/journal.pone.0099176
Interactome profile of the host cellular proteins and the nonstructural protein 2 of porcine reproductive and respiratory syndrome virus
Abstract
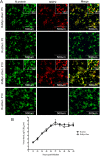
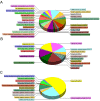
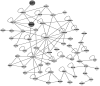
The nonstructural protein 2 (NSP2) is considered to be one of crucial viral proteins in the replication and pathogenesis of porcine reproductive and respiratory syndrome virus (PRRSV). In the present study, the host cellular proteins that interact with the NSP2 of PRRSV were immunoprecipitated with anti-Myc antibody from the MARC-145 cells infected by a recombinant PRRSV with 3xMyc tag insertion in its NSP2-coding region, and then 285 cellular proteins interacting with NSP2 were identified by LC-MS/MS. The Gene Ontology and enriched KEGG Pathway bioinformatics analyses indicated that the identified proteins could be assigned to different subcellular locations and functional classes. Functional analysis of the interactome profile highlighted cellular pathways associated with infectious disease, translation, immune system, nervous system and signal transduction. Two interested cellular proteins-BCL2-associated athanogene 6 (BAG6) and apoptosis-inducing factor 1 (AIF1) which may involve in transporting of NSP2 to Endoplasmic reticulum (ER) or PRRSV-driven apoptosis were validated by Western blot. The interactome data between PRRSV NSP2 and cellular proteins contribute to the understanding of the roles of NSP2 in the replication and pathogenesis of PRRSV, and also provide novel cellular target proteins for elucidating the associated molecular mechanisms of the interaction of host cellular proteins with viral proteins in regulating the viral replication.
Conflict of interest statement
Figures




References
-
- Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, et al. (2005) Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc 227: 385–392. - PubMed
-
- Garner MG, Whan IF, Gard GP, Phillips D (2001) The expected economic impact of selected exotic diseases on the pig industry of Australia. Rev Sci Tech 20: 671–685. - PubMed
-
- Keffaber KK (1989) Reproductive failure of unknown etiology. Am Assoc Swine Pract Newsletter 1: 1–10.
-
- Albina E (1997) Porcine reproductive and respiratory syndrome: ten years of experience (1986–1996) with this undesirable viral infection. Vet Res 28: 305–352. - PubMed
-
- Zhou L, Yang H (2010) Porcine reproductive and respiratory syndrome in China. Virus Res 154: 31–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

