Dusp3 and Psme3 are associated with murine susceptibility to Staphylococcus aureus infection and human sepsis
- PMID: 24901344
- PMCID: PMC4047107
- DOI: 10.1371/journal.ppat.1004149
Dusp3 and Psme3 are associated with murine susceptibility to Staphylococcus aureus infection and human sepsis
Erratum in
- PLoS Pathog. 2014 Jun;10(6):e1004259. Thaden, Joshua [corrected to Thaden, Joshua T]
Abstract
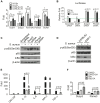
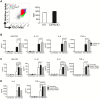
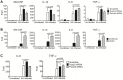
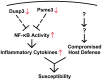
Using A/J mice, which are susceptible to Staphylococcus aureus, we sought to identify genetic determinants of susceptibility to S. aureus, and evaluate their function with regard to S. aureus infection. One QTL region on chromosome 11 containing 422 genes was found to be significantly associated with susceptibility to S. aureus infection. Of these 422 genes, whole genome transcription profiling identified five genes (Dcaf7, Dusp3, Fam134c, Psme3, and Slc4a1) that were significantly differentially expressed in a) S. aureus -infected susceptible (A/J) vs. resistant (C57BL/6J) mice and b) humans with S. aureus blood stream infection vs. healthy subjects. Three of these genes (Dcaf7, Dusp3, and Psme3) were down-regulated in susceptible vs. resistant mice at both pre- and post-infection time points by qPCR. siRNA-mediated knockdown of Dusp3 and Psme3 induced significant increases of cytokine production in S. aureus-challenged RAW264.7 macrophages and bone marrow derived macrophages (BMDMs) through enhancing NF-κB signaling activity. Similar increases in cytokine production and NF-κB activity were also seen in BMDMs from CSS11 (C57BL/6J background with chromosome 11 from A/J), but not C57BL/6J. These findings suggest that Dusp3 and Psme3 contribute to S. aureus infection susceptibility in A/J mice and play a role in human S. aureus infection.
Conflict of interest statement
VGF served as Chair of V710 Scientific Advisory Committee (Merck), has received grant support from Cerexa, Pfizer, Advanced Liquid Logic, MedImmune, has been a paid consultant for Merck, Astellas, Affinium, Bayer, Theravance, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, Medicines Company, Biosynexus, MedImmune, and Inimex, and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis. JL is employed by Quintiles Innovations. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures



References
-
- Bayer AS (1982) Staphylococcal bacteremia and endocarditis: state of the art. Arch Intern Med 142: 1169–1177. - PubMed
-
- Eady EA, Cove JH (2003) Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus–an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis 16: 103–124. - PubMed
-
- Marchant B, Brown J (1987) Toxic shock syndrome and staphylococcal pneumonia. Lancet 2: 578. - PubMed
-
- Verhoef J, Verbrugh HA (1981) Host determinants in staphylococcal disease. Annu Rev Med 32: 107–122. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

