Rosa26-GFP direct repeat (RaDR-GFP) mice reveal tissue- and age-dependence of homologous recombination in mammals in vivo
- PMID: 24901438
- PMCID: PMC4046920
- DOI: 10.1371/journal.pgen.1004299
Rosa26-GFP direct repeat (RaDR-GFP) mice reveal tissue- and age-dependence of homologous recombination in mammals in vivo
Abstract
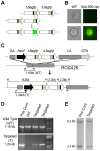
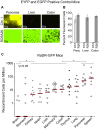
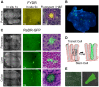
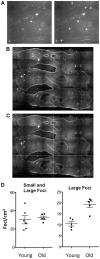
Homologous recombination (HR) is critical for the repair of double strand breaks and broken replication forks. Although HR is mostly error free, inherent or environmental conditions that either suppress or induce HR cause genomic instability. Despite its importance in carcinogenesis, due to limitations in our ability to detect HR in vivo, little is known about HR in mammalian tissues. Here, we describe a mouse model in which a direct repeat HR substrate is targeted to the ubiquitously expressed Rosa26 locus. In the Rosa26 Direct Repeat-GFP (RaDR-GFP) mice, HR between two truncated EGFP expression cassettes can yield a fluorescent signal. In-house image analysis software provides a rapid method for quantifying recombination events within intact tissues, and the frequency of recombinant cells can be evaluated by flow cytometry. A comparison among 11 tissues shows that the frequency of recombinant cells varies by more than two orders of magnitude among tissues, wherein HR in the brain is the lowest. Additionally, de novo recombination events accumulate with age in the colon, showing that this mouse model can be used to study the impact of chronic exposures on genomic stability. Exposure to N-methyl-N-nitrosourea, an alkylating agent similar to the cancer chemotherapeutic temozolomide, shows that the colon, liver and pancreas are susceptible to DNA damage-induced HR. Finally, histological analysis of the underlying cell types reveals that pancreatic acinar cells and liver hepatocytes undergo HR and also that HR can be specifically detected in colonic somatic stem cells. Taken together, the RaDR-GFP mouse model provides new understanding of how tissue and age impact susceptibility to HR, and enables future studies of genetic, environmental and physiological factors that modulate HR in mammals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, et al.. (2006) DNA Repair and Mutagenesis. Washington, DC: ASM Press.
-
- Hoeijmakers JH (2009) DNA damage, aging, and cancer. N Engl J Med 361: 1475–1485. - PubMed
-
- Lieber MR (2008) The mechanism of human nonhomologous DNA end joining. J Biol Chem 283: 1–5. - PubMed
-
- Helleday T, Lo J, van Gent DC, Engelward BP (2007) DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 6: 923–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NIEHS P30-ES002109/ES/NIEHS NIH HHS/United States
- ES007020/ES/NIEHS NIH HHS/United States
- R33 CA112151/CA/NCI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- T32 ES007020/ES/NIEHS NIH HHS/United States
- NCI P30-CA014051/CA/NCI NIH HHS/United States
- P30 ES002109/ES/NIEHS NIH HHS/United States
- NIH P41-EB015871/EB/NIBIB NIH HHS/United States
- Y02 ES007020/ES/NIEHS NIH HHS/United States
- P41 EB015871/EB/NIBIB NIH HHS/United States
- P01 CA026731/CA/NCI NIH HHS/United States
- NIH R33-CA112151/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

