Effect of lanthanide complex structure on cell viability and association
- PMID: 24901440
- PMCID: PMC4060611
- DOI: 10.1021/ic500282n
Effect of lanthanide complex structure on cell viability and association
Abstract
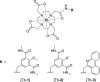
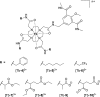
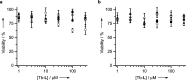
A systematic study of the effect of hydrophobicity and charge on the cell viability and cell association of lanthanide metal complexes is presented. The terbium luminescent probes feature a macrocyclic polyaminocarboxylate ligand (DOTA) in which the hydrophobicity of the antenna and that of the carboxyamide pendant arms are independently varied. Three sensitizing antennas were investigated in terms of their function in vitro: 2-methoxyisophthalamide (IAM(OMe)), 2-hydroxyisophthalamide (IAM), and 6-methylphenanthridine (Phen). Of these complexes, Tb-DOTA-IAM exhibited the highest quantum yield, although the higher cell viability and more facile synthesis of the structurally related Tb-DOTA-IAM(OMe) platform renders it more attractive. Further modification of this latter core structure with carboxyamide arms featuring hydrophobic benzyl, hexyl, and trifluoro groups as well as hydrophilic amino acid based moieties generated a family of complexes that exhibit high cell viability (ED50 > 300 μM) regardless of the lipophilicity or the overall complex charge. Only the hexyl-substituted complex reduced cell viability to 60% in the presence of 100 μM complex. Additionally, cellular association was investigated by ICP-MS and fluorescence microscopy. Surprisingly, the hydrophobic moieties did not increase cell association in comparison to the hydrophilic amino acid derivatives. It is thus postulated that the hydrophilic nature of the 2-methoxyisophthalamide antenna (IAM(OMe)) disfavors the cellular association of these complexes. As such, responsive luminescent probes based on this scaffold would be appropriate for the detection of extracellular species.
Figures



Similar articles
-
Octadentate cages of Tb(III) 2-hydroxyisophthalamides: a new standard for luminescent lanthanide labels.J Am Chem Soc. 2011 Dec 14;133(49):19900-10. doi: 10.1021/ja2079898. Epub 2011 Nov 14. J Am Chem Soc. 2011. PMID: 22010878 Free PMC article.
-
Synthesis and photophysical characterization of highly luminescent silica films doped with substituted 2-hydroxyphthalamide (IAM) terbium complexes.Dalton Trans. 2011 Nov 21;40(43):11530-8. doi: 10.1039/c1dt11131k. Epub 2011 Sep 26. Dalton Trans. 2011. PMID: 21952506
-
Siderophore inspired tetra- and octadentate antenna ligands for luminescent Eu(III) and Tb(III) complexes.J Inorg Biochem. 2016 Sep;162:263-273. doi: 10.1016/j.jinorgbio.2016.01.006. Epub 2016 Jan 9. J Inorg Biochem. 2016. PMID: 26832605
-
Development of responsive lanthanide-based magnetic resonance imaging and luminescent probes for biological applications.Chem Pharm Bull (Tokyo). 2010 Oct;58(10):1283-94. doi: 10.1248/cpb.58.1283. Chem Pharm Bull (Tokyo). 2010. PMID: 20930392 Review.
-
Luminescent Eu(III) and Tb(III) complexes: developing lanthanide luminescent-based devices.J Fluoresc. 2005 Jul;15(4):585-95. doi: 10.1007/s10895-005-2831-9. J Fluoresc. 2005. PMID: 16167216 Review.
Cited by
-
Metallointercalators-DNA Tetrahedron Supramolecular Self-Assemblies with Increased Serum Stability.ACS Nano. 2022 Feb 22;16(2):2928-2941. doi: 10.1021/acsnano.1c10084. Epub 2022 Feb 8. ACS Nano. 2022. PMID: 35133785 Free PMC article.
-
Recent Advances in Luminescence Imaging of Biological Systems Using Lanthanide(III) Luminescent Complexes.Molecules. 2020 Apr 29;25(9):2089. doi: 10.3390/molecules25092089. Molecules. 2020. PMID: 32365719 Free PMC article. Review.
-
Hydrazone Molecular Switches with Paramagnetic Center as 19F Magnetic Resonance Imaging Relaxation Enhancement Agents for pH Imaging.ACS Sens. 2023 May 26;8(5):1971-1979. doi: 10.1021/acssensors.3c00080. Epub 2023 May 17. ACS Sens. 2023. PMID: 37198734 Free PMC article.
-
A Practical Route for the Preparation of 1,4,7-Triazacyclononanyl Diacetates with a Hydroxypyridinonate Pendant Arm.Molecules. 2015 Oct 23;20(10):19393-405. doi: 10.3390/molecules201019393. Molecules. 2015. PMID: 26512638 Free PMC article.
References
-
- Coogan M. P.; Fernandez-Moreira V. Chem. Commun. 2014, 50, 384–399. - PubMed
- Kobayashi H.; Longmire M. R.; Ogawa M.; Choyke P. L. Chem. Soc. Rev. 2011, 40, 4626–4648. - PMC - PubMed
- Lo K. K.-W.; Choi A. W.-T.; Law W. H.-T. Dalton Trans. 2012, 41, 6021–6047. - PubMed
- Baggaley E.; Weinstein J. A.; Williams J. A. G. Coord. Chem. Rev. 2012, 256, 1762–1785.
-
- Lippert A. R.; Gschneidtner T.; Chang C. J. Chem. Commun. 2010, 46, 7510–7512. - PubMed
- Ye Z.; Chen J.; Wang G.; Yuan J. Anal. Chem. 2011, 83, 4163–4169. - PubMed
- Xiao Y.; Ye Z.; Wang G.; Yuan J. Inorg. Chem. 2012, 51, 2940–2946. - PubMed
- Peterson K. L.; Margherio M. J.; Doan P.; Wilke K. T.; Pierre V. C. Inorg. Chem. 2013, 52, 9390–9398. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

