Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients
- PMID: 24901467
- PMCID: PMC8925015
- DOI: 10.1002/14651858.CD010253.pub2
Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients
Abstract
Background: Liver transplantation is an established treatment option for end-stage liver failure. To date, no consensus has been reached on the use of immunosuppressive T-cell antibody induction for preventing rejection after liver transplantation.
Objectives: To assess the benefits and harms of immunosuppressive T-cell specific antibody induction compared with placebo, no induction, or another type of T-cell specific antibody induction for prevention of acute rejection in liver transplant recipients.
Search methods: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index Expanded, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) until September 2013.
Selection criteria: Randomised clinical trials assessing immunosuppression with T-cell specific antibody induction compared with placebo, no induction, or another type of antibody induction in liver transplant recipients. Our inclusion criteria stated that participants within each included trial should have received the same maintenance immunosuppressive therapy. We planned to include trials with all of the different types of T-cell specific antibodies that are or have been used for induction (ie., polyclonal antibodies (rabbit of horse antithymocyte globulin (ATG), or antilymphocyte globulin (ALG)), monoclonal antibodies (muromonab-CD3, anti-CD2, or alemtuzumab), and interleukin-2 receptor antagonists (daclizumab, basiliximab, BT563, or Lo-Tact-1)).
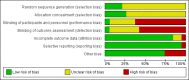
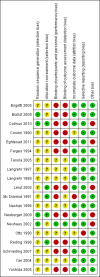
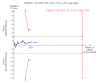
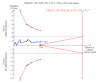
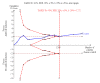
Data collection and analysis: We used RevMan analysis for statistical analysis of dichotomous data with risk ratio (RR) and of continuous data with mean difference (MD), both with 95% confidence intervals (CIs). We assessed the risk of systematic errors (bias) using bias risk domains with definitions. We used trial sequential analysis to control for random errors (play of chance). We presented outcome results in a summary of findings table.
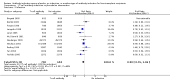
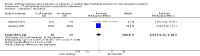
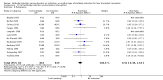
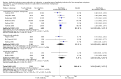
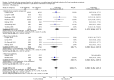
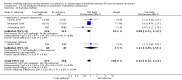
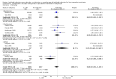
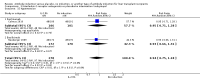
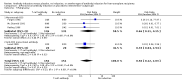
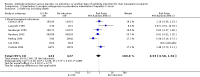
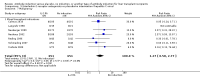
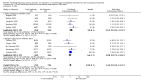
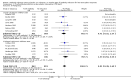
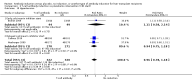
Main results: We included 19 randomised clinical trials with a total of 2067 liver transplant recipients. All 19 trials were with high risk of bias. Of the 19 trials, 16 trials were two-arm trials, and three trials were three-arm trials. Hence, we found 25 trial comparisons with antibody induction agents: interleukin-2 receptor antagonist (IL-2 RA) versus no induction (10 trials with 1454 participants); monoclonal antibody versus no induction (five trials with 398 participants); polyclonal antibody versus no induction (three trials with 145 participants); IL-2 RA versus monoclonal antibody (one trial with 87 participants); and IL-2 RA versus polyclonal antibody (two trials with 112 participants). Thus, we were able to compare T-cell specific antibody induction versus no induction (17 trials with a total of 1955 participants). Overall, no difference in mortality (RR 0.91; 95% CI 0.64 to 1.28; low-quality of evidence), graft loss including death (RR 0.92; 95% CI 0.71 to 1.19; low-quality of evidence), and adverse events ((RR 0.97; 95% CI 0.93 to 1.02; low-quality evidence) outcomes was observed between any kind of T-cell specific antibody induction compared with no induction when the T-cell specific antibody induction agents were analysed together or separately. Acute rejection seemed to be reduced when any kind of T-cell specific antibody induction was compared with no induction (RR 0.85, 95% CI 0.75 to 0.96; moderate-quality evidence), and when trial sequential analysis was applied, the trial sequential monitoring boundary for benefit was crossed before the required information size was obtained. Furthermore, serum creatinine was statistically significantly higher when T-cell specific antibody induction was compared with no induction (MD 3.77 μmol/L, 95% CI 0.33 to 7.21; low-quality evidence), as well as when polyclonal T-cell specific antibody induction was compared with no induction, but this small difference was not clinically significant. We found no statistically significant differences for any of the remaining predefined outcomes - infection, cytomegalovirus infection, hepatitis C recurrence, malignancy, post-transplant lymphoproliferative disease, renal failure requiring dialysis, hyperlipidaemia, diabetes mellitus, and hypertension - when the T-cell specific antibody induction agents were analysed together or separately. Limited data were available for meta-analysis on drug-specific adverse events such as haematological adverse events for antithymocyte globulin. No data were found on quality of life.When T-cell specific antibody induction agents were compared with another type of antibody induction, no statistically significant differences were found for mortality, graft loss, and acute rejection for the separate analyses. When interleukin-2 receptor antagonists were compared with polyclonal T-cell specific antibody induction, drug-related adverse events were less common among participants treated with interleukin-2 receptor antagonists (RR 0.23, 95% CI 0.09 to 0.63; low-quality evidence), but this was caused by the results from one trial, and trial sequential analysis could not exclude random errors. We found no statistically significant differences for any of the remaining predefined outcomes: infection, cytomegalovirus infection, hepatitis C recurrence, malignancy, post-transplant lymphoproliferative disease, renal failure requiring dialysis, hyperlipidaemia, diabetes mellitus, and hypertension. No data were found on quality of life.
Authors' conclusions: The effects of T-cell antibody induction remain uncertain because of the high risk of bias of the randomised clinical trials, the small number of randomised clinical trials reported, and the limited numbers of participants and outcomes in the trials. T-cell specific antibody induction seems to reduce acute rejection when compared with no induction. No other clear benefits or harms were associated with the use of any kind of T-cell specific antibody induction compared with no induction, or when compared with another type of T-cell specific antibody. Hence, more randomised clinical trials are needed to assess the benefits and harms of T-cell specific antibody induction compared with placebo, and compared with another type of antibody, for prevention of rejection in liver transplant recipients. Such trials ought to be conducted with low risks of systematic error (bias) and low risk of random error (play of chance).
Conflict of interest statement
None known.
Figures
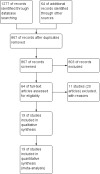






















































































Update of
References
References to studies included in this review
Bogetti 2005 {published data only}
-
- Bogetti D, Jarzembowski T‐M, Sankary H‐N, Manzelli A, Knight P‐S, Chejfec G, et al. Hepatic ischemia/reperfusion injury can be modulated with thymoglobulin induction therapy. Transplantation Proceedings 2005; Vol. 37, issue 1:404‐6. - PubMed
-
- Bogetti D, Sankary HN, Chejfec G, Cotler S, Testa G, Benedetti E. Thymoglobulin (TG) induction protects liver allografts from ischemia/reperfusion injury (IRI). American Journal of Transplantation 2004; Vol. 4, issue Suppl 8:25.
Boillot 2009 {published data only}
-
- Boillot O, Poncet G, Méchet I, Dumortier J, Delafosse B, Sagnard P, et al. Randomized trial of triple based immunosuppression using tacrolimus, mycophenolate mofetil and steroids quadruple regimen induction with thymoglobulin in liver transplantation. Hepatology 2000; Vol. 32, issue 4 (Pt 2):599A.
-
- Boillot O, Seket B, Dumortier J, Pittau G, Boucaud C, Bouffard Y, et al. Thymoglobulin induction in liver transplant recipients with a tacrolimus, mycophenolate mofetil, and steroid immunosuppressive regimen: a five‐year randomized prospective study. Liver Transplantation 2009;15(11):1426‐34. - PubMed
Calmus 2010 {published data only}
-
- Calmus Y, Kamar N, Gugenheim J, Duvoux C, Ducerf C, Wolf P, et al. Assessing renal function with daclizumab induction and delayed tacrolimus introduction in liver transplant recipients. Transplantation 2010;89(12):1504‐10. - PubMed
Cosimi 1990 {published data only}
-
- Cosimi AB, Jenkins RL, Rohrer RJ, Delmonico FL, Hoffman M, Monaco AP. A randomized clinical trial of prophylactic OKT3 monoclonal antibody in liver allograft recipients. Archives of Surgery 1990; Vol. 125, issue 6:781‐4. - PubMed
Eghtesad 2011 {published data only}
-
- Eghtesad B, Forrest T, Fijiki M, Diago T, Hodgkinson P, Hashimoto K, et al. A pilot randomized controlled clinical trial of thymoglobulin (r‐ATG) induction with extended delay of calcineurin inhibitor therapy in liver transplantation ‐ Interim analysis. International Liver Transplant Society. 2011:O‐21.
Farges 1994 {published data only}
-
- Farges O, Ericzon BG, Bresson Hadni S, Lynch SV, Hockerstedt K, Houssin D, et al. A randomized trial of OKT3‐based versus cyclosporine‐based immunoprophylaxis after liver transplantation. Long‐term results of a European and Australian multicenter study. Transplantation 1994; Vol. 58, issue 8:891‐8. - PubMed
-
- Farges O, Ericzon BG, Miguet JP, Lynch S, Hockerstedt K, Chapuis Y, et al. [Immunoprophylaxis with OKT3 after hepatic transplantation: results of a multicenter, prospective, controlled study]. Annales de Chirurgie 1993; Vol. 47, issue 7:675.
Fasola 2005 {published data only}
-
- Fasola CG, Smallwood G, Martinez E, Steiber A, Heffron TG. Daclizumab (DZB) induction in adult hepatitis C (HCV) liver transplant recipients (OLT): a single center experience with different antibody doses. Liver Transplantation 2005;11:C‐44.
Langrehr 1997 {published data only}
-
- Bechstein WO, Langrehr JM, Lohmann R, Lobeck H, Blumhardt G, Neuhaus P. Interleukin‐2 receptor antibody versus ATG for induction immunosuppression after liver transplantation: results of a prospective randomized trial. Langenbecks Archiv für Chirurgie 1994;379:99‐102.
-
- Langrehr J, Guckelberger O, Neumann U, Nussler N, Lobeck H, Lohmann R, et al. A randomized trial comparing quadruple induction therapy with ANTI‐IL‐2 receptor antibody or ATG after liver transplantation, 1997. www.astp.org (accessed 12 April 2012).
-
- Langrehr JM, Guckelberger O, Bechstein WO, Lobeck H, Meuer S, Schlag H, et al. Quadruple induction therapy following liver transplantation with interleukin‐2 receptor antibody BT563 or ATG. A prospective randomized trial. Langenbecks Archiv fur Chirurgie 1997;Suppl 1 Forumband:673‐6.
-
- Langrehr JM, Guckelberger O, Nussler N, Radtke A, Lemmens HP, Jonas S, et al. Interleukin‐2 receptor antibody versus antithymocyte globulin as part of quadruple induction therapy after orthotopic liver transplantation: a randomized study. Transplantation Proceedings 1996; Vol. 28, issue 6:3204. - PubMed
-
- Langrehr JM, Nussler NC, Neumann U, Guckelberger O, Lohmann R, Radtke A, et al. A prospective randomized trial comparing interleukin‐2 receptor antibody versus antithymocyte globulin as part of a quadruple immunosuppressive induction therapy following orthotopic liver transplantation. Transplantation 1997; Vol. 63, issue 12:1772‐81. - PubMed
Langrehr 1998 {published data only}
-
- Glanemann M, Langrehr JM, Raakow R, Guckelberger O, Lohmann R, Klupp J, et al. Anti‐IL‐2 receptor BT563 versus placebo: a randomized trial for induction therapy after liver transplantation. Transplantation Proceedings 1998;30(5):2159‐60. - PubMed
-
- Langrehr JM, Glanemann M, Guckelberger O, Klupp J, Neumann U, Machens C, et al. A randomized, placebo‐controlled trial with anti‐interleukin‐2 receptor antibody for immunosuppressive induction therapy after liver transplantation. Clinical Transplantation 1998;12(4):303‐12. - PubMed
-
- Langrehr JM, Glanemann M, Schneller A, Neumann U, Guckelberger O, Lohmann R, et al. A randomized trial comparing anti‐interleukin‐2 receptor antibody and placebo for immunosuppressive therapy after OLT. Transplantation Proceedings 1998; Vol. 30, issue 4:1445‐6. - PubMed
Lerut 2005 {published data only}
-
- Lerut J, Thuyne V, Mathijs J, Lemaire J, Talpe S, Roggen F, et al. Anti‐CD2 monoclonal antibody and tacrolimus in adult liver transplantation. Transplantation 2005;80:1186‐93. - PubMed
Mc Diarmid 1991 {published data only}
-
- McDiarmid SV, Busuttil RW, Levy P, Millis MJ, Terasaki PI, Ament ME. The long‐term outcome of OKT3 compared with cyclosporine prophylaxis after liver transplantation. Transplantation 1991; Vol. 52, issue 1:91‐7. - PubMed
-
- McDiarmid SV, Millis MJ, Terasaki P, Vargas JH, Ament ME, Busuttil W. Induction of immunosuppression in pediatric orthotopic liver transplantation. Clinical Transplantation 1991;5:174‐80.
-
- McDiarmid SV, Millis MJ, Terasaki PI, Ament ME, Busuttil RW. OKT3 prophylaxis in liver transplantation. Digestive Diseases and Sciences 1991; Vol. 36, issue 10:1418‐26. - PubMed
-
- Millis JM, McDiarmid SV, Hiatt JR, Brems JJ, Colonna JO 2nd, Klein AS, et al. Randomized prospective trial of OKT3 for early prophylaxis of rejection after liver transplantation. Transplantation 1989; Vol. 47, issue 1:82‐8. - PubMed
Nashan 1996 {published data only}
-
- Nashan B, Schlitt HJ, Schwinzer R, Ringe B, Kuse E, Tusch G, et al. Immunoprophylaxis with a monoclonal anti‐IL‐2 receptor antibody in liver transplant patients. Transplantation 1996; Vol. 61, issue 4:546‐54. - PubMed
-
- Nashan B, Schwinzer R, Schlitt HJ, Wonigeit K, Pichlmayr R. Immunological effects of the anti‐IL‐2 receptor monoclonal antibody BT 563 in liver allografted patients. Transplant Immunology 1995;3:203‐11. - PubMed
Neuberger 2009 {published data only}
-
- Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, et al. Delayed introduction of reduced‐dose tacrolimus, and renal function in liver transplantation: the 'ReSpECT' study. American Journal of Transplantation 2009;9(2):327‐36. - PubMed
Neuhaus 2002 {published data only}
-
- Nashan B, Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, et al. The monoclonal anti‐IL 2 receptor antibody basiliximab reduces the incidence and degree of acute rejections after liver transplantation. Zeitschrift fur Gastroenterologie 2000;38:536.
-
- Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, Abeywickrama K, et al. Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: results from a double‐blind randomized placebo‐controlled trial. Liver Transplantation 2002;8:132‐42. - PubMed
-
- Neuhaus P, Nashan B, Clavin PA, Kittur D, Salizzoni M, Rimola A, et al. Basiliximab (Simulect) reduces the rate and severity of acute rejection in adult liver transplant recipients [abstract]. Liver Transplantation 2000; Vol. 6, issue 3:C‐25.
Otto 1996 {published data only}
-
- Otto G, Hofmann WJ, Gaweco AS, Seelos R, Herfarth C, Meuer S. Influence of the anti‐CD25 monoclonal antibody BT563 on clinical and biological rejection after orthotopic liver transplantation. Transplantation Proceedings 1996; Vol. 28, issue 6:3210‐1. - PubMed
Reding 1996 {published data only}
-
- Reding R, Feyaerts A, Vraux H, Latinne D, De‐La Parra B, Cornet A, et al. Prophylactic immunosuppression with anti‐interleukin‐2 receptor monoclonal antibody LO‐Tact‐1 versus OKT3 in liver allografting. A two‐year follow‐up study. Transplantation 1996;61:1406‐9. - PubMed
-
- Reding R, Vraux H, Ville de Goyet J, Sokal E, Hemptinne B, Latinne D, et al. Monoclonal antibodies in prophylactic immunosuppression after liver transplantation. A randomized controlled trial comparing OKT3 and anti‐IL‐2 receptor monoclonal antibody LO‐Tact‐1. Transplantation 1993; Vol. 55, issue 3:534‐41. - PubMed
Schmeding 2007 {published data only}
-
- Schmeding M, Sauer IM, Kiessling A, Pratschke J, Neuhaus R, Neuhaus P, et al. Influence of basiliximab induction therapy on long term outcome after liver transplantation, a prospectively randomised trial. Annals of Transplantation 2007;12(3):15‐21. - PubMed
Yan 2004 {published data only}
-
- Yan LN, Wang WT, Li B, Lu SC, Wen TF. Induction with basiliximab reduces acute rejection in Chinese liver transplant recipients treated with cyclosporin, steroids and MMF. Liver Transplantation 2004;10:C‐5.
Yoshida 2005 {published data only}
-
- Yoshida EM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Cantarovich M, et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low‐dose tacrolimus regimen vs. a standard‐dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transplantation 2005;11(9):1064‐72. - PubMed
References to studies excluded from this review
Benitez 2010 {published data only}
-
- Benitez CE, Puig‐Pey I, Lopez M, Martinez‐Llordella M, Lozano JJ, Bohne F, et al. ATG‐Fresenius treatment and low‐dose tacrolimus: results of a randomized controlled trial in liver transplantation. American Journal of Transplantation 2010;10:2296‐304. - PubMed
Boillot 2005 {published data only}
-
- Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, et al. Corticosteroid‐free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transplantation 2005; Vol. 11, issue 1:61‐7. - PubMed
De Simone 2007 {published data only}
-
- Simone P, Carlis L, Filipponi F, Grazi GL, Cuomo O, Santaniello W, et al. Results of a multicenter, randomized open‐label, controlled clinical trial comparing basiliximab versus steroids in hepatitis C positive liver transplant patients. Transplant International 2007;20(Suppl 2):33.
-
- Simone P, Carlis L, Grazi GL, Cuomo O, Calise F, Castagneto M, et al. Results of a multicenter, randomized open‐label trial comparing basiliximab vs. steroids in HCV liver transplant patients. American Journal of Transplantation 2007;7(Suppl 2):312.
Eason 2003 {published data only}
-
- Eason JD, Blazek J, Mason A, Loss GE. Steroid‐free immunosuppression through thymoglobulin induction in liver transplantation: results of a prospective randomized trial. Transplantation Proceedings 2000;32(4):208A. - PubMed
-
- Eason JD, Loss GE, Blazek J, Nair S, Mason AL. Steroid‐free liver transplantation using rabbit antithymocyte globulin induction: results of a prospective randomized trial. Liver Transplantation 2001;7(8):693‐7. - PubMed
-
- Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE Jr. Steroid‐free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation 2003;75(8):1396‐9. - PubMed
-
- Nair S, Loss GE, Cohen AJ, Eason JD. Induction with rabbit antithymocyte globulin versus induction with corticosteroids in liver transplantation: impact on recurrent hepatitis C virus infection. Transplantation 2006;81:620‐2. - PubMed
Kato 2001 {published data only}
-
- Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, et al. Randomized trial of steroid‐free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation 2007;84:829‐35. - PubMed
-
- Kato T, Neff GW, Montalbano M, Hung O, Lavandera R, Levi D, et al. Steroid‐free induction with daclizumab and tacrolimus in liver transplant recipients with hepatitis C: a preliminary report [abstract]. Hepatology 2001;34:362A.
Kato 2007 {published data only}
-
- Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, et al. Randomized trial of steroid‐free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation 2007;84:829‐35. - PubMed
-
- Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid‐free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Hepatology 2005; Vol. 37, issue 2:1217‐9. - PubMed
Klintmalm 2011 {published data only}
-
- Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, et al. A randomized, multicenter study comparing steroid‐free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transplantation 2011;17(12):1394‐403. - PubMed
-
- Klintmalm GB, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, et al. Corticosteroid‐free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1‐year interim results of the HCV‐3 study. Liver Transplantation 2007;13(11):1521‐31. - PubMed
Lupo 2008 {published data only}
-
- Lupo L, Panzera P, Tandoi F, Carbotta G, Giannelli G, Santantonio T, et al. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: a prospective randomized clinical trial. Transplantation 2008;86(7):925‐31. - PubMed
-
- Lupo L, Ricci P, Caputi L, Tandoi F, Aquilino F, Palma G, et al. Basiliximab vs steroids in liver transplantation immunosuppression. A prospective randomized clinical trial. Liver Transplantation 2005;11(7):LB17.
Neumann 2012 {published data only}
Spada 2006 {published data only}
-
- Spada M, Bertani A, Petz W, Torri E, Sonzogni A, Guizzetti M, et al. A randomized trial for tacrolimus and steroids vs. tacrolimus and basiliximab in pediatric liver transplantation. Hepatology 2004; Vol. 40, issue 4 Suppl 1:473A.
-
- Spada M, Petz W, Bertani A, Riva S, Sonzogni A, Giovannelli M, et al. Randomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppression. American Journal of Transplantation 2006; Vol. 6, issue 8:1913‐21. - PubMed
Washburn 2001 {published data only}
-
- Washburn K, Speeg KV, Esterl R, Cigarroa F, Pollack M, Tourtellot C, et al. Steroid elimination 24 hours after liver transplantation using daclizumab, tacrolimus, and mycophenolate mofetil. Transplantation 2001;72(10):1675‐9. - PubMed
Additional references
Adams 1992
-
- Adams DH, Neuberger JM. Treatment of acute rejection. Seminars in Liver Disease 1992;12(1):80‐8. - PubMed
Banff 1997
-
- Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 1997; Vol. 25, issue 3:658‐63. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Chatenoud 2008
-
- Chatenoud L. The long and winding road towards induction of allograft tolerance in the clinic. Transplant International 2008;21(8):725‐7. - PubMed
Chen 2006
-
- Chen W, Zhang L. Regulatory T‐cell subsets and their roles in transplantation tolerance. Current Opinion in Organ Transplantation 2006;11(4):373‐8.
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ 2011 (accessed 19 March 2014).
Dienstag 2012
-
- Dienstag JL, Cosimi AB. Liver transplantation ‐ a vision realized. New England Journal of Medicine 2012;367(16):1483‐5. - PubMed
Egger 1997
FK506 1994a
-
- The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. New England Journal of Medicine 1994;331(17):1110‐5. - PubMed
FK506 1994b
-
- European FK506 Multicentre Liver Study Group. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet 1994;344(8920):423‐8. - PubMed
Flechner 2008
-
- Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor‐sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clinical Transplantation 2008;22(1):1‐15. - PubMed
Gluud 2014
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2014, Issue 1. Art. No.: LIVER.
Goralczyk 2011
-
- Goralczyk AD, Hauke N, Bari N, Tsui TY, Lorf T, Obed A. Interleukin 2 receptor antagonists for liver transplant recipients: a systematic review and meta‐analysis of controlled studies. Hepatology 2011;54(2):541‐54. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania, USA: Barnett International/PAREXEL, 1997.
Iversen 2009
-
- Iversen M, Corris P. Immunosuppression. In: Fisher AJ, Verleden G, Massard G editor(s). European Respiratory Monthly: Lung Transplantation. Plymouth, UK: European Respiratory Society Journals Ltd, 2009:147‐68.
Keus 2009
-
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [MEDLINE: ] - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lechler 2005
-
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation ‐ how much of the promise has been realized?. Nature Medicine 2005;11(6):605‐13. - PubMed
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Magliocca 2006
-
- Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath‐1H) for immunosuppressive therapy in organ transplantation. Transplant International 2006;19(9):705‐14. - PubMed
Marcos 2004
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Moser 2002
-
- Moser MA. Options for induction immunosuppression in liver transplant recipients. Drugs 2002;62(7):995‐1011. - PubMed
Neuhaus 1993
-
- Neuhaus P, Bechstein WO, Blumhardt G, Wiens M, Lemmens P, Langrehr JM, et al. Comparison of quadruple immunosuppression after liver transplantation with ATG or IL‐2 receptor antibody. Transplantation 1993;55(6):1320‐7. - PubMed
O'Grady 2002
-
- O'Grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet 2002;360(9340):1119‐25. - PubMed
OPTN 2009
-
- OPTN / SRTR Annual Report. The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. www.ustransplant.org/annual_reports/current/ 2009 (accessed 19 March 2014).
Penninga 2012
Penninga 2013
Penninga 2013a
Penninga 2014
Penninga 2014a
Perera 2009
-
- Perera MT, Mirza DF, Elias E. Liver transplantation: issues for the next 20 years. Journal of Gastroenterology and Hepatology 2009;24(Suppl 3):S124‐S131. - PubMed
Pillai 2009
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savović 2012
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Seyfert‐Margolis 2010
-
- Seyfert‐Margolis V, Feng S. Tolerance: is it achievable in pediatric solid organ transplantation?. Pediatric Clinics of North America 2010;57:523‐38. - PubMed
Sgourakis 2009
-
- Sgourakis G, Radtke A, Fouzas I, Mylona S, Goumas K, Gockel I, et al. Corticosteroid‐free immunosuppression in liver transplantation: a meta‐analysis and meta‐regression of outcomes. Transplant International 2009;22(9):892‐905. - PubMed
SPIRIT 2013
SPIRIT 2013a
Starzl 2003
Starzl 2008
Sweeting 2004
-
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [MEDLINE: ] - PubMed
Thompson 2002
-
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2011
Thorlund 2011a
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 19 March 2014).
Transplant Activity Report 2009
-
- Transplant activity report 2008/2009 UK. www.organdonation.nhs.uk/ukt/statistics/statistics.jsp 2009 (accessed 19 March 2014).
Uemura 2011
-
- Uemura T, Schaefer E, Hollenbeak CS, Khan A, Kadry Z. Outcome of induction immunosuppression for liver transplantation comparing anti‐thymocyte globulin, daclizumab, and corticosteroid. Transplant International 2011;24(7):640‐50. - PubMed
Wang 2010
-
- Wang XF, Li JD, Peng Y, Dai Y, Shi G, Xu W. Interleukin‐2 receptor antagonists in liver transplantation: a meta‐analysis of randomized trials. Transplantation Proceedings 2010;42(10):4567‐72. - PubMed
Webster 2010
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wiesner 2003
-
- Wiesner RH, Rakela J, Ishitani MB, Mulligan DC, Spivey JR, Steers JL, et al. Recent advances in liver transplantation. Mayo Clinic Proceedings 2003; Vol. 78, issue 2:197‐210. - PubMed
References to other published versions of this review
Penninga 2012a
-
- Penninga L, Wettergren A, Wilson CH, Steinbrüchel DA, Gluud C. Immunosuppressive T cell antibody induction therapy for liver transplant recipients. Cochrane Database of Systematic Reviews 2012, Issue 11, 2011 ‐ 10, 2012. [DOI: 10.1002/14651858.CD007341.pub2] - DOI
Wilson 2008
-
- Wilson CH, Asher JF, Manas DM. Immunosuppressive T cell antibodies for liver transplant recipients. Cochrane Database of Systematic Reviews 2011, Issue 3, 2008 ‐ 10, 2011. [DOI: 10.1002/14651858.CD007341] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

