Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability
- PMID: 24901524
- PMCID: PMC4047110
- DOI: 10.1371/journal.pone.0099256
Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability
Abstract
Background: Lactulose mannitol ratio tests are clinically useful for assessing disorders characterised by changes in gut permeability and for assessing mixing in the intestinal lumen. Variations between currently used test protocols preclude meaningful comparisons between studies. We determined the optimal sampling period and related this to intestinal residence.
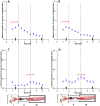
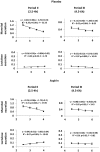
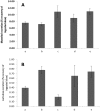
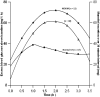
Methods: Half-hourly lactulose and mannitol urinary excretions were determined over 6 hours in 40 healthy female volunteers after administration of either 600 mg aspirin or placebo, in randomised order at weekly intervals. Gastric and small intestinal transit times were assessed by the SmartPill in 6 subjects from the same population. Half-hourly percentage recoveries of lactulose and mannitol were grouped on a basis of compartment transit time. The rate of increase or decrease of each sugar within each group was explored by simple linear regression to assess the optimal period of sampling.
Key results: The between subject standard errors for each half-hourly lactulose and mannitol excretion were lowest, the correlation of the quantity of each sugar excreted with time was optimal and the difference between the two sugars in this temporal relationship maximal during the period from 2½-4 h after ingestion. Half-hourly lactulose excretions were generally increased after dosage with aspirin whilst those of mannitol were unchanged as was the temporal pattern and period of lowest between subject standard error for both sugars.
Conclusion: The results indicate that between subject variation in the percentage excretion of the two sugars would be minimised and the differences in the temporal patterns of excretion would be maximised if the period of collection of urine used in clinical tests of small intestinal permeability were restricted to 2½-4 h post dosage. This period corresponds to a period when the column of digesta column containing the probes is passing from the small to the large intestine.
Conflict of interest statement
Figures
Similar articles
-
The effect of aspirin and smoking on urinary excretion profiles of lactulose and mannitol in young women: toward a dynamic, aspirin augmented, test of gut mucosal permeability.Neurogastroenterol Motil. 2012 Sep;24(9):e401-11. doi: 10.1111/j.1365-2982.2012.01969.x. Epub 2012 Jul 4. Neurogastroenterol Motil. 2012. PMID: 22757650
-
Differential trafficking of saccharidic probes following aspirin in clinical tests of intestinal permeability in young healthy women.Clin Exp Pharmacol Physiol. 2014 Feb;41(2):107-17. doi: 10.1111/1440-1681.12163. Clin Exp Pharmacol Physiol. 2014. PMID: 24033480 Clinical Trial.
-
Assessment of the Effect of Intestinal Permeability Probes (Lactulose And Mannitol) and Other Liquids on Digesta Residence Times in Various Segments of the Gut Determined by Wireless Motility Capsule: A Randomised Controlled Trial.PLoS One. 2015 Dec 2;10(12):e0143690. doi: 10.1371/journal.pone.0143690. eCollection 2015. PLoS One. 2015. PMID: 26629926 Free PMC article. Clinical Trial.
-
Ascorbic Acid may Exacerbate Aspirin-Induced Increase in Intestinal Permeability.Basic Clin Pharmacol Toxicol. 2015 Sep;117(3):195-203. doi: 10.1111/bcpt.12388. Epub 2015 Mar 4. Basic Clin Pharmacol Toxicol. 2015. PMID: 25641731 Clinical Trial.
-
Urinary excretion of gluten immunoreactive peptides as an indicator of gastrointestinal function after fasting and dietary provocation in healthy volunteers.Front Immunol. 2024 Aug 5;15:1433304. doi: 10.3389/fimmu.2024.1433304. eCollection 2024. Front Immunol. 2024. PMID: 39161759 Free PMC article.
Cited by
-
Investigating the association between the symptoms of women with Fibromyalgia, Digestive function, and markers of the microbiota of the Gastrointestinal Tract (The FIDGIT Study): study protocol.BMC Musculoskelet Disord. 2023 Feb 27;24(1):150. doi: 10.1186/s12891-023-06259-3. BMC Musculoskelet Disord. 2023. PMID: 36849949 Free PMC article.
-
A Simple, Robust, and Convenient HPLC Assay for Urinary Lactulose and Mannitol in the Dual Sugar Absorption Test.Molecules. 2022 Apr 21;27(9):2677. doi: 10.3390/molecules27092677. Molecules. 2022. PMID: 35566024 Free PMC article.
-
The praziquantel in preschoolers (PIP) trial: study protocol for a phase II PK/PD-driven randomised controlled trial of praziquantel in children under 4 years of age.Trials. 2021 Sep 6;22(1):601. doi: 10.1186/s13063-021-05558-1. Trials. 2021. PMID: 34488846 Free PMC article.
-
Small Intestinal Permeability and Metabolomic Profiles in Feces and Plasma Associate With Clinical Response in Patients With Active Psoriatic Arthritis Participating in a Fecal Microbiota Transplantation Trial: Exploratory Findings From the FLORA Trial.ACR Open Rheumatol. 2023 Nov;5(11):583-593. doi: 10.1002/acr2.11604. Epub 2023 Sep 22. ACR Open Rheumatol. 2023. PMID: 37736702 Free PMC article.
-
Associations of poor water, sanitation, and hygiene and parasite burden with markers of environmental enteric dysfunction in preschool-age children infected with Schistosoma mansoni in Uganda.Trop Med Int Health. 2025 Jan;30(1):14-21. doi: 10.1111/tmi.14061. Epub 2024 Dec 1. Trop Med Int Health. 2025. PMID: 39618064
References
-
- Halme L, Turunen U, Tuominen J, Forsstrom T, Turpeinen U (2000) Comparison of iohexol and lactulose-mannitol tests as markers of disease activity in patients with inflammatory bowel disease. Scandinavian Journal of Clinical & Laboratory Investigation 60: 695–701. - PubMed
-
- Vogelsang H, Wyatt J, Penner E, Lochs H (1995) Screening for celiac disease in first-degree relatives of patients with celiac disease by lactulose/mannitol test. The American journal of gastroenterology 90: 1838–1842. - PubMed
-
- Dastych M, Novotná H, Číhalová J (2008) Lactulose/Mannitol Test and Specificity, Sensitivity, and Area under Curve of Intestinal Permeability Parameters in Patients with Liver Cirrhosis and Crohn’s Disease. Digestive Diseases and Sciences 53: 2789–2792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

