Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants
- PMID: 24901968
- PMCID: PMC4046925
- DOI: 10.1371/journal.pgen.1004406
Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants
Abstract
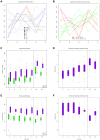
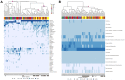
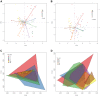
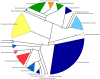
In spite of its major impact on life-long health, the process of microbial succession in the gut of infants remains poorly understood. Here, we analyze the patterns of taxonomic and functional change in the gut microbiota during the first year of life for a birth cohort of 13 infants. We detect that individual instances of gut colonization vary in the temporal dynamics of microbiota richness, diversity, and composition at both functional and taxonomic levels. Nevertheless, trends discernible in a majority of infants indicate that gut colonization occurs in two distinct phases of succession, separated by the introduction of solid foods to the diet. This change in resource availability causes a sharp decrease in the taxonomic richness of the microbiota due to the loss of rare taxa (p = 2.06e-9), although the number of core genera shared by all infants increases substantially. Moreover, although the gut microbial succession is not strictly deterministic, we detect an overarching directionality of change through time towards the taxonomic and functional composition of the maternal microbiota. Succession is however not complete by the one year mark, as significant differences remain between one-year-olds and their mothers in terms of taxonomic (p = 0.009) and functional (p = 0.004) microbiota composition, and in taxonomic richness (p = 2.76e-37) and diversity (p = 0.016). Our results also indicate that the taxonomic composition of the microbiota shapes its functional capacities. Therefore, the observed inter-individual variability in taxonomic composition during succession is not fully compensated by functional equivalence among bacterial genera and may have important physiological consequences. Finally, network analyses suggest that positive interactions among core genera during community assembly contribute to ensure their permanence within the gut, and highlight an expansion of complexity in the interactions network as the core of taxa shared by all infants grows following the introduction of solid foods.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Sekirov I, Russell SL, Antunes LC, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90: 859–904. - PubMed
-
- Collado MC, D'Auria G, Mira A, Francino MP (2013) Human Microbiome and Diseases: A Metagenomic Approach. In: Watson RR and Preedy VR, editors. Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease. San Diego: Academic Press. pp. 235–249.
-
- Baas-Becking L (1934) Geobiologie of inleiding tot de milieukunde. Van Stockum & Zoon. 263 p.
-
- de Wit R, Bouvier T (2006) ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ Microbiol 8: 755–758. - PubMed
-
- McConnell EL, Basit AW, Murdan S (2008) Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol 60: 63–70. - PubMed

