Back to the future with phenotypic screening
- PMID: 24902068
- PMCID: PMC4102969
- DOI: 10.1021/cn500051h
Back to the future with phenotypic screening
Abstract
There are no disease-modifying drugs for any old age associated neurodegenerative disease or stroke. This is at least in part due to the failure of drug developers to recognize that the vast majority of neurodegenerative diseases arise from a confluence of multiple toxic insults that accumulate during normal aging and interact with genetic and environmental risk factors. Thus, it is unlikely that the current single target approach based upon rare dominant mutations or even a few preselected targets is going to yield useful drugs for these conditions. Therefore, the identification of drug candidates for neurodegeneration should be based upon their efficacy in phenotypic screening assays that reflect the biology of the aging brain, not a single, preselected target. It is argued here that this approach to drug discovery is the most likely to produce safe and effective drugs for neurodegenerative diseases.
Figures

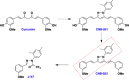

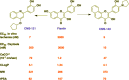
References
-
- Pangalos M. N.; Schechter L. E.; Hurko O. (2007) Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discovery 6, 521–532. - PubMed
-
- Zhang H. Y.; Chen L. L.; Li X. J.; Zhang J. (2010) Evolutionary inspirations for drug discovery. Trends Pharmacol. Sci. 31, 443–448. - PubMed
-
- Swinney D. C.; Anthony J. (2011) How were new medicines discovered?. Nat. Rev. Drug Discovery 10, 507–519. - PubMed
-
- Munos B. (2009) Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discovery 8, 959–968. - PubMed
- Paul S. M.; Mytelka D. S.; Dunwiddie C. T.; Persinger C. C.; Munos B. H.; Lindborg S. R.; Schacht A. L. (2010) How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discovery 9, 203–214. - PubMed
-
- Newman D. J.; Cragg G. M.; Snader K. M. (2003) Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 66, 1022–1037. - PubMed
- Newman D. J.; Cragg G. M. (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

