Ryanodine receptors: physiological function and deregulation in Alzheimer disease
- PMID: 24902695
- PMCID: PMC4063224
- DOI: 10.1186/1750-1326-9-21
Ryanodine receptors: physiological function and deregulation in Alzheimer disease
Abstract
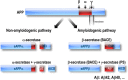
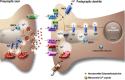
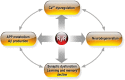
Perturbed Endoplasmic Reticulum (ER) calcium (Ca2+) homeostasis emerges as a central player in Alzheimer disease (AD). Accordingly, different studies have reported alterations of the expression and the function of Ryanodine Receptors (RyR) in human AD-affected brains, in cells expressing familial AD-linked mutations on the β amyloid precursor protein (βAPP) and presenilins (the catalytic core in γ-secretase complexes cleaving the βAPP, thereby generating amyloid β (Aβ) peptides), as well as in the brain of various transgenic AD mice models. Data converge to suggest that RyR expression and function alteration are associated to AD pathogenesis through the control of: i) βAPP processing and Aβ peptide production, ii) neuronal death; iii) synaptic function; and iv) memory and learning abilities. In this review, we document the network of evidences suggesting that RyR could play a complex dual "compensatory/protective versus pathogenic" role contributing to the setting of histopathological lesions and synaptic deficits that are associated with the disease stages. We also discuss the possible mechanisms underlying RyR expression and function alterations in AD. Finally, we review recent publications showing that drug-targeting blockade of RyR and genetic manipulation of RyR reduces Aβ production, stabilizes synaptic transmission, and prevents learning and memory deficits in various AD mouse models. Chemically-designed RyR "modulators" could therefore be envisioned as new therapeutic compounds able to delay or block the progression of AD.
Figures
References
-
- Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer’s disease. J Neurochem. 1995;65:1431–1444. - PubMed
-
- Wolfe MS. Processive proteolysis by gamma-secretase and the mechanism of Alzheimer’s disease. Biol Chem. 2012;393:899–905. - PubMed
-
- St George-Hyslop PH, Tanzi RE, Haines JL, Polinsky RJ, Farrer L, Myers RH, Gusella JF. Molecular genetics of familial Alzheimer’s disease. Eur Neurol. 1989;29(Suppl 3):25–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

