Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade
- PMID: 24903021
- PMCID: PMC4097121
- DOI: 10.1158/2326-6066.CIR-13-0215
Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade
Abstract
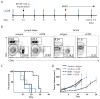
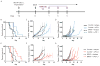
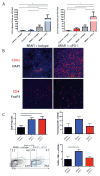
BRAF-targeted therapy results in objective responses in the majority of patients; however, the responses are short lived (∼6 months). In contrast, treatment with immune checkpoint inhibitors results in a lower response rate, but the responses tend to be more durable. BRAF inhibition results in a more favorable tumor microenvironment in patients, with an increase in CD8(+) T-cell infiltrate and a decrease in immunosuppressive cytokines. There is also increased expression of the immunomodulatory molecule PDL1, which may contribute to the resistance. On the basis of these findings, we hypothesized that BRAF-targeted therapy may synergize with the PD1 pathway blockade to enhance antitumor immunity. To test this hypothesis, we developed a BRAF(V600E)/Pten(-/-) syngeneic tumor graft immunocompetent mouse model in which BRAF inhibition leads to a significant increase in the intratumoral CD8(+) T-cell density and cytokine production, similar to the effects of BRAF inhibition in patients. In this model, CD8(+) T cells were found to play a critical role in the therapeutic effect of BRAF inhibition. Administration of anti-PD1 or anti-PDL1 together with a BRAF inhibitor led to an enhanced response, significantly prolonging survival and slowing tumor growth, as well as significantly increasing the number and activity of tumor-infiltrating lymphocytes. These results demonstrate synergy between combined BRAF-targeted therapy and immune checkpoint blockade. Although clinical trials combining these two strategies are ongoing, important questions still remain unanswered. Further studies using this new melanoma mouse model may provide therapeutic insights, including optimal timing and sequence of therapy.
©2014 American Association for Cancer Research.
Conflict of interest statement
A. Sharpe has licensed patents and received patent royalties from Genentech and CoStim Pharmaceuticals. G. Freeman has patents on the PD-1 pathway from Bristol-Myers-Squibb, Genentech, Merck, EMD-Serrono, Boehringer-Ingelheim, Amplimmune, and CoStim. F. S. Hodi has served as a non-paid consultant to Genentech/Roche, Merck, and Bristol-Myers Squibb and received clinical trial support from Genentech, Merck, and Bristol-Myers Squibb. K. Flaherty has served as a consultant to GlaxoSmithKline and Genentech/Roche.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA163125/CA/NCI NIH HHS/United States
- P01AI056299/AI/NIAID NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- P30CA016672/CA/NCI NIH HHS/United States
- P01 CA163222/CA/NCI NIH HHS/United States
- R01 CA176839/CA/NCI NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- 5T32HL007627-28/HL/NHLBI NIH HHS/United States
- R01 AR043369/AR/NIAMS NIH HHS/United States
- 1K08CA160692-01A1/CA/NCI NIH HHS/United States
- L30 CA162156/CA/NCI NIH HHS/United States
- P50CA101942/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- P50 CA101942/CA/NCI NIH HHS/United States
- T32 HL007627/HL/NHLBI NIH HHS/United States
- K08 CA160692/CA/NCI NIH HHS/United States
- U54CA163125/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

