Effects of JSOG-6 on protection against bone loss in ovariectomized mice through regulation of osteoblast differentiation and osteoclast formation
- PMID: 24903150
- PMCID: PMC4066836
- DOI: 10.1186/1472-6882-14-184
Effects of JSOG-6 on protection against bone loss in ovariectomized mice through regulation of osteoblast differentiation and osteoclast formation
Abstract
Background: JSOG-6 is used as a traditional medicine to relieve the symptoms associated with inflammation, rheumatism, and osteoporosis in Korea. In the present study, we investigated the effects of JSOG-6 on bone loss prevention both in in vitro and in vivo as well as its underlying mechanism of action.
Methods: Protection against bone loss was assessed in an ovariectomized (OVX) mouse model. Bone microarchitecture was measured using a micro-computed tomography to detect the parameters of three-dimensional structure of a trabecular bone. Serum biomarkers were also evaluated in an OVX-induced model. Osteoclasts derived from mouse bone marrow cells (BMCs) and osteoblastic MC3T3-E1 cells were also employed to investigate the mechanism of action.
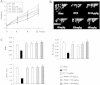
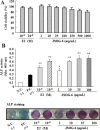
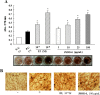
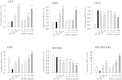
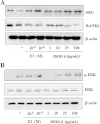
Results: Oral administration of JSOG-6 significantly increased the bone mineral density (BMD) of the femur in OVX mice in vivo. Especially, the reduced Tb.No (trabecular bone number) in the OVX group was significantly recovered by JSOG-6 treatment. The serum levels of alkaline phosphatase (ALP), osteocalcin, C-terminal telopeptide, and tartrate-resistant acid phosphatase, biomarkers of bone resorption, were significantly elevated in OVX mice, but JSOG-6 effectively inhibited the increase in OVX mice. JSOG-6 was also found to enhance the osteoblastic differentiation and maturation with the increase of the density and ALP activity, a marker of osteoblastic differentiation, as well as calcium deposition, a marker of osteoblastic maturation in MC3T3-E1 cells. The effects of JSOG-6 on osteoblastic differentiation were also associated in part with the increase of ALP and OPN mRNA expressions and the decrease of RANKL mRNA expression in MC3T3-E1 cells.
Conclusions: The findings demonstrate that JSOG-6 induced protection against bone loss in OVX mice, and its anti-osteoporotic property might be, in part, a function of the stimulation of osteoblast differentiation and the inhibition of osteoclast formation. These findings suggest that JSOG-6 might be an applicable therapeutic traditional medicine for the regulation of the osteoporotic response.
Figures

References
-
- Alexander JM, Bab I, Fish S, Müller R, Uchiyama T, Gronowicz G, Nahounou M, Zhao Q, White DW, Chorev M, Gazit D, Rosenblatt M. Human parathyroid hormone 1–34 reverses bone loss in ovariectomized mice. J Bone Miner Res. 2001;161:665–673. - PubMed
-
- Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;15:349–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

