Dual catalysis. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis
- PMID: 24903560
- PMCID: PMC4406487
- DOI: 10.1126/science.1253647
Dual catalysis. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis
Abstract
The routine application of C(sp3)-hybridized nucleophiles in cross-coupling reactions remains an unsolved challenge in organic chemistry. The sluggish transmetalation rates observed for the preferred organoboron reagents in such transformations are a consequence of the two-electron mechanism underlying the standard catalytic approach. We describe a mechanistically distinct single-electron transfer-based strategy for the activation of organoboron reagents toward transmetalation that exhibits complementary reactivity patterns. Application of an iridium photoredox catalyst in tandem with a nickel catalyst effects the cross-coupling of potassium alkoxyalkyl- and benzyltrifluoroborates with an array of aryl bromides under exceptionally mild conditions (visible light, ambient temperature, no strong base). The transformation has been extended to the asymmetric and stereoconvergent cross-coupling of a secondary benzyltrifluoroborate.
Copyright © 2014, American Association for the Advancement of Science.
Figures
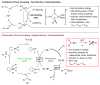

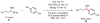
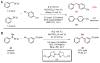
Comment in
-
Catalysis. Self-control tames the coupling of reactive radicals.Science. 2014 Jul 25;345(6195):381-2. doi: 10.1126/science.1256755. Epub 2014 Jul 24. Science. 2014. PMID: 25061190 No abstract available.
References
-
- de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. 2. Wiley-VCH; Weinheim, Germany: 2004.
-
- Hartwig JF. Organotransition Metal Chemistry: From Bonding to Catalysis. University Science; Sausalito, CA: 2010.
-
- Rudolph A, Lautens M. Angew Chem Int Ed. 2009;48:2656–2670. - PubMed
-
- Hegedus LS, Soderberg BCG. Transition Metals in the Synthesis of Complex Organic Molecules. 3. University Science; Sausalito, CA: 2010.
-
- Doucet H. Eur J Org Chem. 2008;2008:2013–2030.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

