Dual catalysis. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp³-carbons with aryl halides
- PMID: 24903563
- PMCID: PMC4296524
- DOI: 10.1126/science.1255525
Dual catalysis. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp³-carbons with aryl halides
Abstract
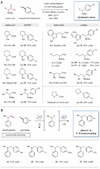
Over the past 40 years, transition metal catalysis has enabled bond formation between aryl and olefinic (sp(2)) carbons in a selective and predictable manner with high functional group tolerance. Couplings involving alkyl (sp(3)) carbons have proven more challenging. Here, we demonstrate that the synergistic combination of photoredox catalysis and nickel catalysis provides an alternative cross-coupling paradigm, in which simple and readily available organic molecules can be systematically used as coupling partners. By using this photoredox-metal catalysis approach, we have achieved a direct decarboxylative sp(3)-sp(2) cross-coupling of amino acids, as well as α-O- or phenyl-substituted carboxylic acids, with aryl halides. Moreover, this mode of catalysis can be applied to direct cross-coupling of C(sp³)-H in dimethylaniline with aryl halides via C-H functionalization.
Copyright © 2014, American Association for the Advancement of Science.
Figures



Comment in
-
Catalysis. Self-control tames the coupling of reactive radicals.Science. 2014 Jul 25;345(6195):381-2. doi: 10.1126/science.1256755. Epub 2014 Jul 24. Science. 2014. PMID: 25061190 No abstract available.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

