Glycoprotein hormones and their receptors emerged at the origin of metazoans
- PMID: 24904013
- PMCID: PMC4079206
- DOI: 10.1093/gbe/evu118
Glycoprotein hormones and their receptors emerged at the origin of metazoans
Abstract
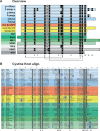
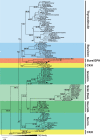
The cystine knot growth factor (CKGF) superfamily includes important secreted developmental regulators, including the families of transforming growth factor beta, nerve growth factor, platelet-derived growth factor, and the glycoprotein hormones (GPHs). The evolutionary origin of the GPHs and the related invertebrate bursicon hormone, and their characteristic receptors, contributes to an understanding of the endocrine system in metazoans. Using a sensitive search method with hidden Markov models, we identified homologs of the hormones and receptors, along with the closely related bone morphogenetic protein (BMP) antagonists in basal metazoans. In sponges and a comb jelly, cystine knot hormones (CKHs) with mixed features of GPHs, bursicon, and BMP antagonists were identified using primary sequence and phylogenetic analysis. Also, we identified potential receptors for these CKHs, leucine-rich repeat-containing G protein-coupled receptors (LGRs), in the same species. Cnidarians, such as the sea anemone, coral, and hydra, diverged later in metazoan evolution and appear to have duplicated and differentiated CKH-like peptides resulting in bursicon/GPH-like peptides and several BMP antagonists: Gremlin (Grem), sclerostin domain containing (SOSD), neuroblastoma suppressor of tumorigenicity 1 (NBL1), and Norrie disease protein. An expanded cnidarian LGR group also evolved, including receptors for GPH and bursicon. With the appearance of bilaterians, a separate GPH (thyrostimulin) along with bursicon and BMP antagonists were present. Synteny indicates that the GPHs, Grem, and SOSD have been maintained in a common gene neighborhood throughout much of metazoan evolution. The stable and highly conserved CKGFs are not identified in nonmetazoan organisms but are established with their receptors in the basal metazoans, becoming critical to growth, development, and regulation in all animals.
Keywords: BMP antagonist; GPH evolution; LGR evolution; bursicon; cystine knot growth factor; thyrostimulin.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures





References
-
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. - PubMed
-
- Avsian-Kretchmer O, Hsueh AJW. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol Endocrinol. 2004;18:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

