Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma
- PMID: 24904120
- PMCID: PMC4468583
- DOI: 10.1182/blood-2014-01-548941
Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma
Abstract
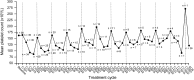
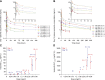
Proteasome inhibition is an effective treatment strategy for multiple myeloma. With improving survival, attention is increasingly focusing on ease of administration and toxicity profile. Ixazomib is an investigational, orally bioavailable 20S proteasome inhibitor. Sixty patients with relapsed and/or refractory multiple myeloma were enrolled on this phase 1 trial to evaluate safety and tolerability and determine the maximum tolerated dose (MTD) of single-agent, oral ixazomib given weekly for 3 of 4 weeks. Upon MTD determination, patients were enrolled to 4 different cohorts based on relapsed/refractory status and prior bortezomib and carfilzomib exposure. The MTD was determined to be 2.97 mg/m(2). Dose-limiting toxicities were grade 3 nausea, vomiting, and diarrhea in 2 patients, and grade 3 skin rash in 1 patient. Common drug-related adverse events were thrombocytopenia (43%), diarrhea (38%), nausea (38%), fatigue (37%), and vomiting (35%). The observed rate of peripheral neuropathy was 20%, with only 1 grade 3 event reported. Nine (18%) patients achieved a partial response or better, including 8 of 30 (27%) evaluable patients treated at the MTD. Pharmacokinetic studies suggested a long terminal half-life of 3.6 to 11.3 days, supporting once-weekly dosing. This trial was registered at www.clinicaltrials.gov as #NCT00963820.
© 2014 by The American Society of Hematology.
Figures


Comment in
-
Oral therapy for multiple myeloma: ixazomib arriving soon.Blood. 2014 Aug 14;124(7):986-7. doi: 10.1182/blood-2014-06-581611. Blood. 2014. PMID: 25124778 Free PMC article.
References
-
- Kumar S. Multiple myeloma - current issues and controversies. Cancer Treat Rev. 2010;36(Suppl 2):S3-11. - PubMed
-
- Richardson PG, Laubach J, Mitsiades C, et al. Tailoring treatment for multiple myeloma patients with relapsed and refractory disease. Oncology (Williston Park) 2010;24(3 Suppl 2):22–29. - PubMed
-
- Gentile M, Recchia AG, Mazzone C, Morabito F. Emerging biological insights and novel treatment strategies in multiple myeloma. Expert Opin Emerg Drugs. 2012;17(3):407–438. - PubMed
-
- Moreau P. The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. Semin Hematol. 2012;49(Suppl 1):S33-S46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

