Structural basis for a pH-sensitive calcium leak across membranes
- PMID: 24904158
- PMCID: PMC4119810
- DOI: 10.1126/science.1252043
Structural basis for a pH-sensitive calcium leak across membranes
Abstract
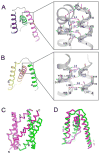
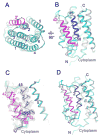
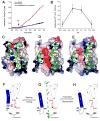
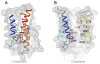
Calcium homeostasis balances passive calcium leak and active calcium uptake. Human Bax inhibitor-1 (hBI-1) is an antiapoptotic protein that mediates a calcium leak and is representative of a highly conserved and widely distributed family, the transmembrane Bax inhibitor motif (TMBIM) proteins. Here, we present crystal structures of a bacterial homolog and characterize its calcium leak activity. The structure has a seven-transmembrane-helix fold that features two triple-helix sandwiches wrapped around a central C-terminal helix. Structures obtained in closed and open conformations are reversibly interconvertible by change of pH. A hydrogen-bonded, pKa (where Ka is the acid dissociation constant)-perturbed pair of conserved aspartate residues explains the pH dependence of this transition, and biochemical studies show that pH regulates calcium influx in proteoliposomes. Homology models for hBI-1 provide insights into TMBIM-mediated calcium leak and cytoprotective activity.
Copyright © 2014, American Association for the Advancement of Science.
Figures
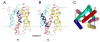
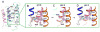
References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Smaili SS, Pereira GJ, Costa MM, Rocha KK, Rodrigues L, et al. The role of calcium stores in apoptosis and autophagy. Curr Mol Med. 2013;13:252–265. - PubMed
-
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. - PubMed
-
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

