Sleep promotes branch-specific formation of dendritic spines after learning
- PMID: 24904169
- PMCID: PMC4447313
- DOI: 10.1126/science.1249098
Sleep promotes branch-specific formation of dendritic spines after learning
Abstract
How sleep helps learning and memory remains unknown. We report in mouse motor cortex that sleep after motor learning promotes the formation of postsynaptic dendritic spines on a subset of branches of individual layer V pyramidal neurons. New spines are formed on different sets of dendritic branches in response to different learning tasks and are protected from being eliminated when multiple tasks are learned. Neurons activated during learning of a motor task are reactivated during subsequent non-rapid eye movement sleep, and disrupting this neuronal reactivation prevents branch-specific spine formation. These findings indicate that sleep has a key role in promoting learning-dependent synapse formation and maintenance on selected dendritic branches, which contribute to memory storage.
Copyright © 2014, American Association for the Advancement of Science.
Figures
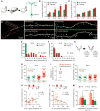
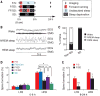


Comment in
-
Neuroscience. Memories--getting wired during sleep.Science. 2014 Jun 6;344(6188):1087-8. doi: 10.1126/science.1255649. Science. 2014. PMID: 24904140 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

