Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles
- PMID: 24904211
- PMCID: PMC4039420
- DOI: 10.2147/IJN.S60270
Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles
Abstract
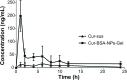
This study aimed to optimize and evaluate a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles (Cur-BSA-NPs-Gel). Albumin nanoparticles were prepared via a desolvation method, and the gels were prepared via a cold method. The central composite design and response surface method was used to evaluate the effects of varying Pluronic F127 and Pluronic F68 concentrations on the sol-gel transition temperature, which is an indicator of optimum formulations. The optimized formulation was a free-flowing liquid below 30.9°C that transformed into a semi-solid gel above 34.2°C after dilution with simulated tear fluid. Results of the in vitro release and erosion behavior study indicated that Cur-BSA-NPs-Gel achieved superior sustained-release effects and that incorporation of albumin nanoparticles exerted minimal effects on the gel structure. In addition, in vivo ophthalmic experiments employing Cur-BSA-NPs-Gel were subsequently performed in rabbits. In vivo eye irritation results showed that Cur-BSA-NPs-Gel might be considered safe for ophthalmic drug delivery. The in vivo study also revealed that the formulation could significantly increase curcumin bioavailability in the aqueous humor. In conclusion, the optimized in situ gel formulation developed in this work has significant potential for ocular application.
Keywords: desolvation method; diabetic retinopathy; ocular drug delivery; sustained release.
Figures


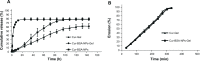
Similar articles
-
Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels.Int J Pharm. 2011 Jun 15;411(1-2):128-35. doi: 10.1016/j.ijpharm.2011.03.054. Epub 2011 Apr 1. Int J Pharm. 2011. PMID: 21459137
-
Formulation and development of ophthalmic in situ gel for the treatment ocular inflammation and infection using application of quality by design concept.Drug Dev Ind Pharm. 2016 Sep;42(9):1406-23. doi: 10.3109/03639045.2015.1137306. Epub 2016 Jan 29. Drug Dev Ind Pharm. 2016. PMID: 26716613
-
Thermosensitive in situ nanogel as ophthalmic delivery system of curcumin: development, characterization, in vitro permeation and in vivo pharmacokinetic studies.Pharm Dev Technol. 2016 Aug;21(5):576-82. doi: 10.3109/10837450.2015.1026607. Epub 2015 May 29. Pharm Dev Technol. 2016. PMID: 26024239
-
Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications.Drug Discov Today. 2019 Aug;24(8):1575-1586. doi: 10.1016/j.drudis.2019.05.036. Epub 2019 Jun 5. Drug Discov Today. 2019. PMID: 31175956 Review.
-
In situ gel systems as 'smart' carriers for sustained ocular drug delivery.Expert Opin Drug Deliv. 2012 Apr;9(4):383-402. doi: 10.1517/17425247.2012.665367. Expert Opin Drug Deliv. 2012. PMID: 22432690 Review.
Cited by
-
Poloxamer Hydrogels for Biomedical Applications.Pharmaceutics. 2019 Dec 10;11(12):671. doi: 10.3390/pharmaceutics11120671. Pharmaceutics. 2019. PMID: 31835628 Free PMC article. Review.
-
Potential of Stimuli-Responsive In Situ Gel System for Sustained Ocular Drug Delivery: Recent Progress and Contemporary Research.Polymers (Basel). 2021 Apr 20;13(8):1340. doi: 10.3390/polym13081340. Polymers (Basel). 2021. PMID: 33923900 Free PMC article. Review.
-
β-Caryophyllene/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Improves Cognitive Deficits in Rats with Vascular Dementia through the Cannabinoid Receptor Type 2 -Mediated Pathway.Front Pharmacol. 2017 Jan 19;8:2. doi: 10.3389/fphar.2017.00002. eCollection 2017. Front Pharmacol. 2017. PMID: 28154534 Free PMC article.
-
Therapeutic Applications of Curcumin Nanoformulations.AAPS J. 2015 Nov;17(6):1341-56. doi: 10.1208/s12248-015-9811-z. Epub 2015 Sep 3. AAPS J. 2015. PMID: 26335307 Free PMC article. Review.
-
Synthesis, characterization, and in vitro evaluation of curcumin-loaded albumin nanoparticles surface-functionalized with glycyrrhetinic acid.Int J Nanomedicine. 2015 Aug 27;10:5475-87. doi: 10.2147/IJN.S88253. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26346750 Free PMC article.
References
-
- Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57(4):347–370. - PubMed
-
- Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complications. 2012;26(1):56–64. - PubMed
-
- Agrawal SS, Naqvi S, Gupta SK, Srivastava S. Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanisms. Food Chem Toxicol. 2012;50(9):3126–3132. - PubMed
-
- Bartlett HE, Eperjesi F. Nutritional supplementation for type 2 diabetes: a systematic review. Ophthalmic Physiol Opt. 2008;28(6):503–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

