Closed-loop control of spinal cord stimulation to restore hand function after paralysis
- PMID: 24904251
- PMCID: PMC4032985
- DOI: 10.3389/fnins.2014.00087
Closed-loop control of spinal cord stimulation to restore hand function after paralysis
Abstract
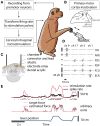
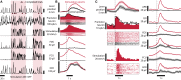
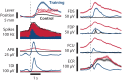
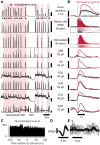
As yet, no cure exists for upper-limb paralysis resulting from the damage to motor pathways after spinal cord injury or stroke. Recently, neural activity from the motor cortex of paralyzed individuals has been used to control the movements of a robot arm but restoring function to patients' actual limbs remains a considerable challenge. Previously we have shown that electrical stimulation of the cervical spinal cord in anesthetized monkeys can elicit functional upper-limb movements like reaching and grasping. Here we show that stimulation can be controlled using cortical activity in awake animals to bypass disruption of the corticospinal system, restoring their ability to perform a simple upper-limb task. Monkeys were trained to grasp and pull a spring-loaded handle. After temporary paralysis of the hand was induced by reversible inactivation of primary motor cortex using muscimol, grasp-related single-unit activity from the ventral premotor cortex was converted into stimulation patterns delivered in real-time to the cervical spinal gray matter. During periods of closed-loop stimulation, task-modulated electromyogram, movement amplitude, and task success rate were improved relative to interleaved control periods without stimulation. In some sessions, single motor unit activity from weakly active muscles was also used successfully to control stimulation. These results are the first use of a neural prosthesis to improve the hand function of primates after motor cortex disruption, and demonstrate the potential for closed-loop cortical control of spinal cord stimulation to reanimate paralyzed limbs.
Keywords: brain-computer interface; closed-loop neuro-prosthetics devices; electrical stimulation; grasp; hand movements; intraspinal microstimulation; non-human primate model; spinal cord injury.
Figures

References
-
- Craggs M. D. (1975). Cortical control of motor prostheses: using the cord-transected baboon as the primate model for human paraplegia. Adv. Neurol. 10, 91–101 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

