Cellular function and pathological role of ATP13A2 and related P-type transport ATPases in Parkinson's disease and other neurological disorders
- PMID: 24904274
- PMCID: PMC4033846
- DOI: 10.3389/fnmol.2014.00048
Cellular function and pathological role of ATP13A2 and related P-type transport ATPases in Parkinson's disease and other neurological disorders
Abstract
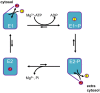
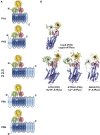
Mutations in ATP13A2 lead to Kufor-Rakeb syndrome, a parkinsonism with dementia. ATP13A2 belongs to the P-type transport ATPases, a large family of primary active transporters that exert vital cellular functions. However, the cellular function and transported substrate of ATP13A2 remain unknown. To discuss the role of ATP13A2 in neurodegeneration, we first provide a short description of the architecture and transport mechanism of P-type transport ATPases. Then, we briefly highlight key P-type ATPases involved in neuronal disorders such as the copper transporters ATP7A (Menkes disease), ATP7B (Wilson disease), the Na(+)/K(+)-ATPases ATP1A2 (familial hemiplegic migraine) and ATP1A3 (rapid-onset dystonia parkinsonism). Finally, we review the recent literature of ATP13A2 and discuss ATP13A2's putative cellular function in the light of what is known concerning the functions of other, better-studied P-type ATPases. We critically review the available data concerning the role of ATP13A2 in heavy metal transport and propose a possible alternative hypothesis that ATP13A2 might be a flippase. As a flippase, ATP13A2 may transport an organic molecule, such as a lipid or a peptide, from one membrane leaflet to the other. A flippase might control local lipid dynamics during vesicle formation and membrane fusion events.
Keywords: alpha-synuclein; dystonia; flippase; heavy metal toxicity; lysosome; mitochondria; mitophagy; parkinsonism.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

