The sodium leak channel, NALCN, in health and disease
- PMID: 24904279
- PMCID: PMC4033012
- DOI: 10.3389/fncel.2014.00132
The sodium leak channel, NALCN, in health and disease
Abstract
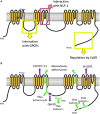
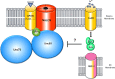
Ion channels are crucial components of cellular excitability and are involved in many neurological diseases. This review focuses on the sodium leak, G protein-coupled receptors (GPCRs)-activated NALCN channel that is predominantly expressed in neurons where it regulates the resting membrane potential and neuronal excitability. NALCN is part of a complex that includes not only GPCRs, but also UNC-79, UNC-80, NLF-1 and src family of Tyrosine kinases (SFKs). There is growing evidence that the NALCN channelosome critically regulates its ion conduction. Both in mammals and invertebrates, animal models revealed an involvement in many processes such as locomotor behaviors, sensitivity to volatile anesthetics, and respiratory rhythms. There is also evidence that alteration in this NALCN channelosome can cause a wide variety of diseases. Indeed, mutations in the NALCN gene were identified in Infantile Neuroaxonal Dystrophy (INAD) patients, as well as in patients with an Autosomal Recessive Syndrome with severe hypotonia, speech impairment, and cognitive delay. Deletions in NALCN gene were also reported in diseases such as 13q syndrome. In addition, genes encoding NALCN, NLF- 1, UNC-79, and UNC-80 proteins may be susceptibility loci for several diseases including bipolar disorder, schizophrenia, Alzheimer's disease, autism, epilepsy, alcoholism, cardiac diseases and cancer. Although the physiological role of the NALCN channelosome is poorly understood, its involvement in human diseases should foster interest for drug development in the near future. Toward this goal, we review here the current knowledge on the NALCN channelosome in physiology and diseases.
Keywords: NALCN; UNC-79; UNC-80; excitability; ion channel.
Figures
References
-
- Anney R. J., Lasky-Su J., O'Dushlaine C., Kenny E., Neale B. M., Mulligan A., et al. (2008). Conduct disorder and ADHD: evaluation of conduct problems as a categorical and quantitative trait in the international multicentre ADHD genetics study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 1369–1378 10.1002/ajmg.b.30871 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

