Heterozygous ambra1 deficiency in mice: a genetic trait with autism-like behavior restricted to the female gender
- PMID: 24904333
- PMCID: PMC4032889
- DOI: 10.3389/fnbeh.2014.00181
Heterozygous ambra1 deficiency in mice: a genetic trait with autism-like behavior restricted to the female gender
Abstract
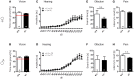
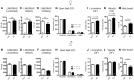
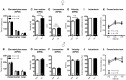
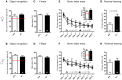
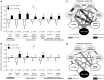
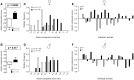
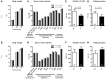
Autism-spectrum disorders (ASD) are heterogeneous, highly heritable neurodevelopmental conditions affecting around 0.5% of the population across cultures, with a male/female ratio of approximately 4:1. Phenotypically, ASD are characterized by social interaction and communication deficits, restricted interests, repetitive behaviors, and reduced cognitive flexibility. Identified causes converge at the level of the synapse, ranging from mutation of synaptic genes to quantitative alterations in synaptic protein expression, e.g., through compromised transcriptional or translational control. We wondered whether reduced turnover and degradation of synapses, due to deregulated autophagy, would lead to similar phenotypical consequences. Ambra1, strongly expressed in cortex, hippocampus, and striatum, is a positive regulator of Beclin1, a principal player in autophagosome formation. While homozygosity of the Ambra1 null mutation causes embryonic lethality, heterozygous mice with reduced Ambra1 expression are viable, reproduce normally, and lack any immediately obvious phenotype. Surprisingly, comprehensive behavioral characterization of these mice revealed an autism-like phenotype in Ambra1 (+/-) females only, including compromised communication and social interactions, a tendency of enhanced stereotypies/repetitive behaviors, and impaired cognitive flexibility. Reduced ultrasound communication was found in adults as well as pups, which achieved otherwise normal neurodevelopmental milestones. These features were all absent in male Ambra1 (+/-) mice. As a first hint explaining this gender difference, we found a much stronger reduction of Ambra1 protein in the cortex of Ambra1 (+/-) females compared to males. To conclude, Ambra1 deficiency can induce an autism-like phenotype. The restriction to the female gender of autism-generation by a defined genetic trait is unique thus far and warrants further investigation.
Keywords: Ambra1; autism composite score; autophagy; cognitive rigidity; heterozygous null mutant mice; repetitive behavior; social interaction; ultrasound communication.
Figures



References
-
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Fifth edition
-
- Asperger H. (1944). The “autistic psychopathy” in childhood. Arch. Psychiatr. Nervenkr. 117, 76–13610.1007/BF01837709 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

