A neurobiological hypothesis of treatment-resistant depression - mechanisms for selective serotonin reuptake inhibitor non-efficacy
- PMID: 24904340
- PMCID: PMC4033019
- DOI: 10.3389/fnbeh.2014.00189
A neurobiological hypothesis of treatment-resistant depression - mechanisms for selective serotonin reuptake inhibitor non-efficacy
Abstract
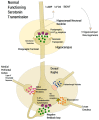
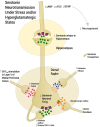
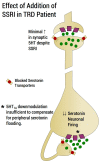
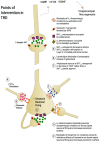
First-line treatment of major depression includes administration of a selective serotonin reuptake inhibitor (SSRI), yet studies suggest that remission rates following two trials of an SSRI are <50%. The authors examine the putative biological substrates underlying "treatment resistant depression (TRD)" with the goal of elucidating novel rationales to treat TRD. We look at relevant articles from the preclinical and clinical literature combined with clinical exposure to TRD patients. A major focus was to outline pathophysiological mechanisms whereby the serotonin system becomes impervious to the desired enhancement of serotonin neurotransmission by SSRIs. A complementary focus was to dissect neurotransmitter systems, which serve to inhibit the dorsal raphe. We propose, based on a body of translational studies, TRD may not represent a simple serotonin deficit state but rather an excess of midbrain peri-raphe serotonin and subsequent deficit at key fronto-limbic projection sites, with ultimate compromise in serotonin-mediated neuroplasticity. Glutamate, serotonin, noradrenaline, and histamine are activated by stress and exert an inhibitory effect on serotonin outflow, in part by "flooding" 5-HT1A autoreceptors by serotonin itself. Certain factors putatively exacerbate this scenario - presence of the short arm of the serotonin transporter gene, early-life adversity and comorbid bipolar disorder - each of which has been associated with SSRI-treatment resistance. By utilizing an incremental approach, we provide a system for treating the TRD patient based on a strategy of rescuing serotonin neurotransmission from a state of SSRI-induced dorsal raphe stasis. This calls for "stacked" interventions, with an SSRI base, targeting, if necessary, the glutamatergic, serotonergic, noradrenergic, and histaminergic systems, thereby successively eliminating the inhibitory effects each are capable of exerting on serotonin neurons. Future studies are recommended to test this biologically based approach for treatment of TRD.
Keywords: dorsal raphe; glutamate; hippocampus; lamotrigine; selective serotonin reuptake inhibitors; somatodendritic 5-HT1A autoreceptors; treatment-resistant depression; α2-heteroreceptors.
Figures
References
-
- Akiskal H. S., Akiskal K. K., Lancrenon S., Hantouche E. G., Fraud J. P., Gury C., et al. (2006). Validating the bipolar spectrum in the French National EPIDEP Study: overview of the phenomenology and relative prevalence of its clinical prototypes. J. Affect. Disord. 96, 197–205 10.1016/j.jad.2006.05.015 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

