Organic electrode coatings for next-generation neural interfaces
- PMID: 24904405
- PMCID: PMC4034607
- DOI: 10.3389/fneng.2014.00015
Organic electrode coatings for next-generation neural interfaces
Abstract
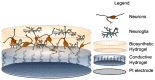
Traditional neuronal interfaces utilize metallic electrodes which in recent years have reached a plateau in terms of the ability to provide safe stimulation at high resolution or rather with high densities of microelectrodes with improved spatial selectivity. To achieve higher resolution it has become clear that reducing the size of electrodes is required to enable higher electrode counts from the implant device. The limitations of interfacing electrodes including low charge injection limits, mechanical mismatch and foreign body response can be addressed through the use of organic electrode coatings which typically provide a softer, more roughened surface to enable both improved charge transfer and lower mechanical mismatch with neural tissue. Coating electrodes with conductive polymers or carbon nanotubes offers a substantial increase in charge transfer area compared to conventional platinum electrodes. These organic conductors provide safe electrical stimulation of tissue while avoiding undesirable chemical reactions and cell damage. However, the mechanical properties of conductive polymers are not ideal, as they are quite brittle. Hydrogel polymers present a versatile coating option for electrodes as they can be chemically modified to provide a soft and conductive scaffold. However, the in vivo chronic inflammatory response of these conductive hydrogels remains unknown. A more recent approach proposes tissue engineering the electrode interface through the use of encapsulated neurons within hydrogel coatings. This approach may provide a method for activating tissue at the cellular scale, however, several technological challenges must be addressed to demonstrate feasibility of this innovative idea. The review focuses on the various organic coatings which have been investigated to improve neural interface electrodes.
Keywords: carbon nanotubes; coatings; conductive polymers; hydrogels; living electrodes; material properties.
Figures





References
-
- Abidian M. R., Martin D. C. (2009). Multifunctional nanobiomaterials for neural interfaces. Adv. Funct. Mater. 19 573–585 10.1002/adfm.200801473 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

