PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating
- PMID: 24904429
- PMCID: PMC4034418
- DOI: 10.3389/fphys.2014.00195
PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating
Abstract
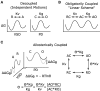
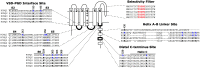
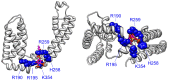
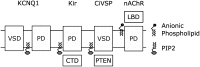
Voltage-gated potassium (Kv) channels contain voltage-sensing (VSD) and pore-gate (PGD) structural domains. During voltage-dependent gating, conformational changes in the two domains are coupled giving rise to voltage-dependent opening of the channel. In addition to membrane voltage, KCNQ (Kv7) channel opening requires the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2). Recent studies suggest that PIP2 serves as a cofactor to mediate VSD-PGD coupling in KCNQ1 channels. In this review, we put these findings in the context of the current understanding of voltage-dependent gating, lipid modulation of Kv channel activation, and PIP2-regulation of KCNQ channels. We suggest that lipid-mediated coupling of functional domains is a common mechanism among KCNQ channels that may be applicable to other Kv channels and membrane proteins.
Keywords: KCNQ; PIP2; ion channel; lipid modulations; voltage-gating.
Figures
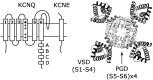
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

